BONDING Ionic Bonding Chemical bonds are electrostatic forces
BONDING Ionic Bonding

Chemical bonds are electrostatic forces that hold atoms together in compounds. Why do atoms form bonds at all? ENERGY! systems of lower energy tend to be favoured over systems of higher energy greater stability Bond energy is the energy required to break a chemical bond and separate the atoms. Bond length is the distance between the nuclei of the bonded atoms and represents the most stable (lowest) energy state.

Ionic bonding Ionic compounds result when a bond between two oppositely charged ions (cations and anions) arises from the transfer of electrons. The force of attraction between the oppositely charged ions constitutes an ionic bond. Ionic bonding occurs between atoms that have large differences in electronegativity. The units of an ionic bond cannot be separated easily by direct heating of the crystal salts. The ions that make up the ionic solid are arranged in a specific array of repeating units (lattice structure). Because of large differences in electronegativity, the atoms in an ionic compound usually come from the s- and d- block metals and the p-block non-metals.
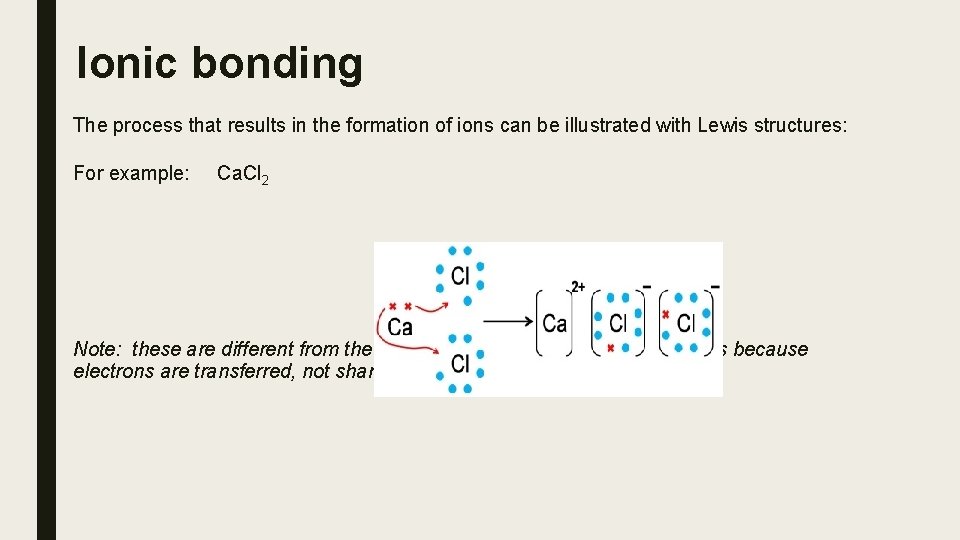
Ionic bonding The process that results in the formation of ions can be illustrated with Lewis structures: For example: Ca. Cl 2 Note: these are different from the Lewis diagrams of covalent compounds because electrons are transferred, not shared
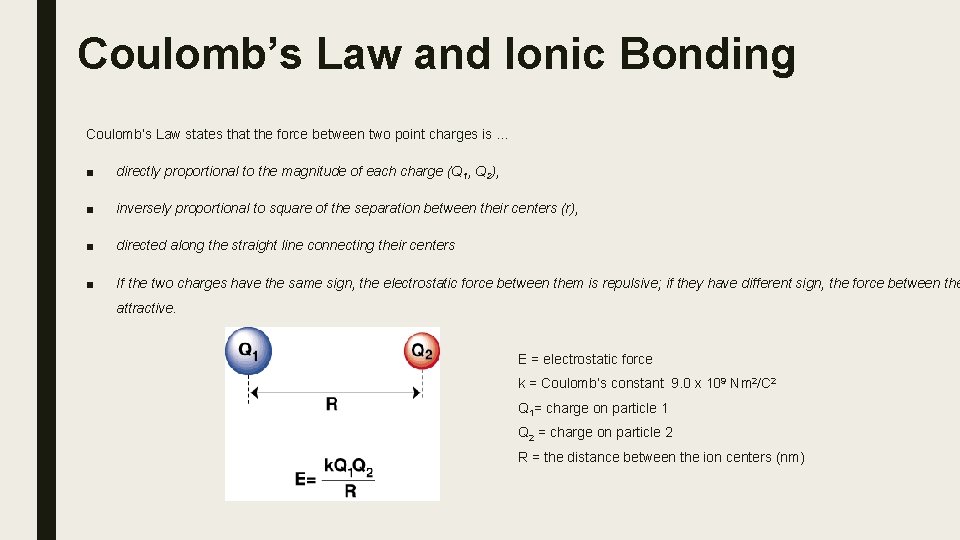
Coulomb’s Law and Ionic Bonding Coulomb’s Law states that the force between two point charges is … ■ directly proportional to the magnitude of each charge (Q 1, Q 2), ■ inversely proportional to square of the separation between their centers (r), ■ directed along the straight line connecting their centers ■ If the two charges have the same sign, the electrostatic force between them is repulsive; if they have different sign, the force between the attractive. E = electrostatic force k = Coulomb’s constant 9. 0 x 109 Nm 2/C 2 Q 1= charge on particle 1 Q 2 = charge on particle 2 R = the distance between the ion centers (nm)
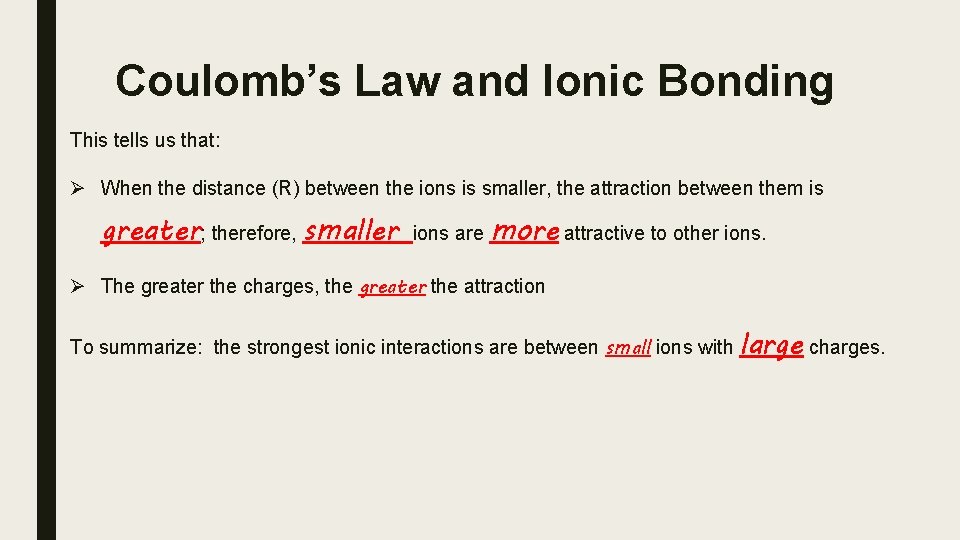
Coulomb’s Law and Ionic Bonding This tells us that: Ø When the distance (R) between the ions is smaller, the attraction between them is greater; therefore, smaller ions are more attractive to other ions. Ø The greater the charges, the greater the attraction To summarize: the strongest ionic interactions are between small ions with large charges.

Properties of Ionic Solids ■ Crystalline with smooth, shiny surfaces ■ Hard but brittle ■ Non-conductors of electricity and heat (in solid form) ■ High melting points

AP Practice Questions
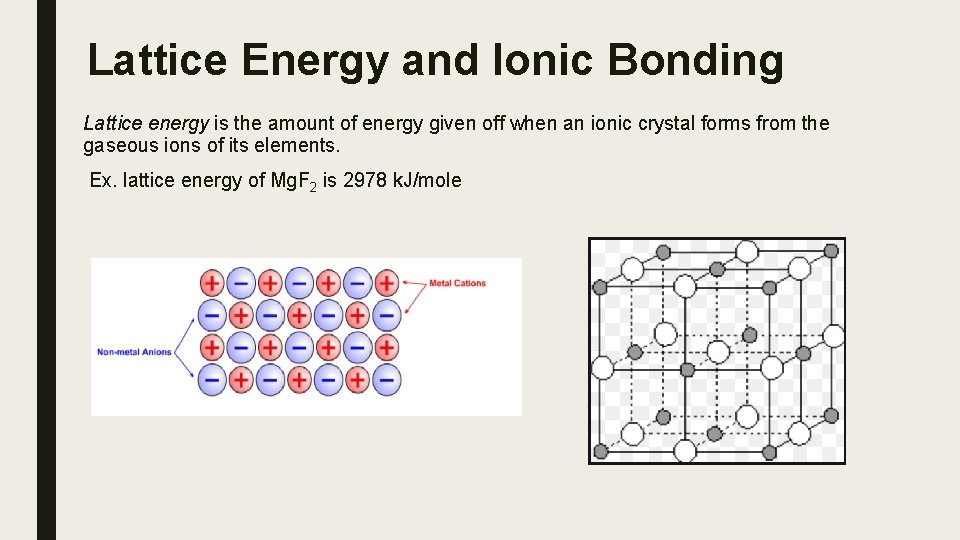
Lattice Energy and Ionic Bonding Lattice energy is the amount of energy given off when an ionic crystal forms from the gaseous ions of its elements. Ex. lattice energy of Mg. F 2 is 2978 k. J/mole

AP Practice Question
- Slides: 10