Bonding Ionic Bonding 1 An ionic bond is
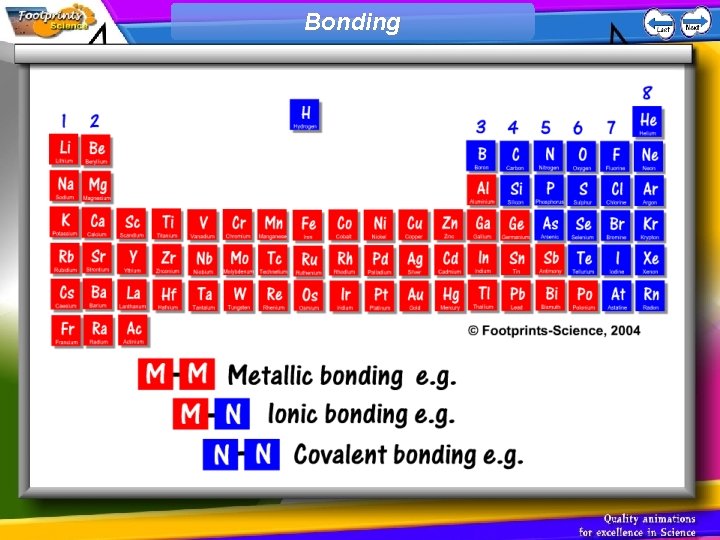
Bonding

Ionic Bonding 1. An ionic bond is formed when a metal atom and a non-metal atom combine 2. The metal atom donates an electron to the non-metal atom 3. The metal atom becomes a positively charged ion, the non-metal atom becomes a negatively charged ion 4. The positive ion attracts the negative ion – an ionic bond
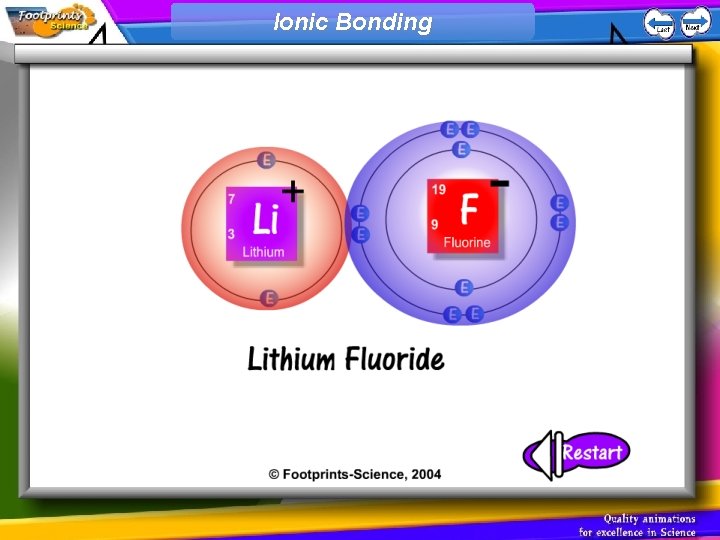
Ionic Bonding
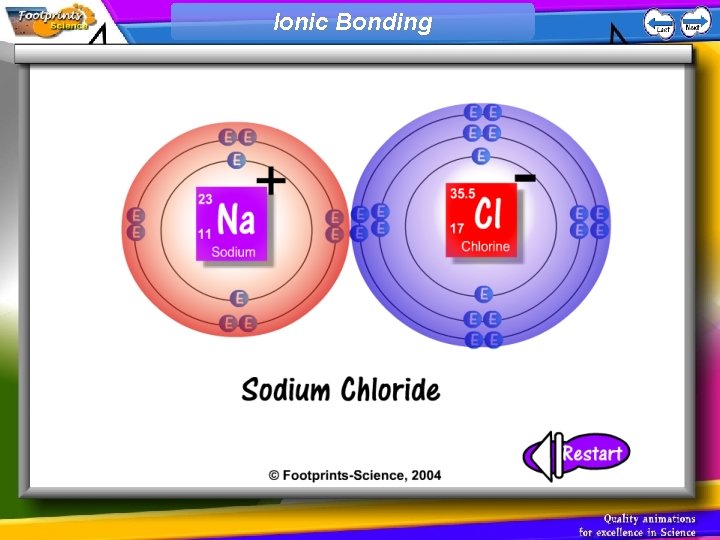
Ionic Bonding
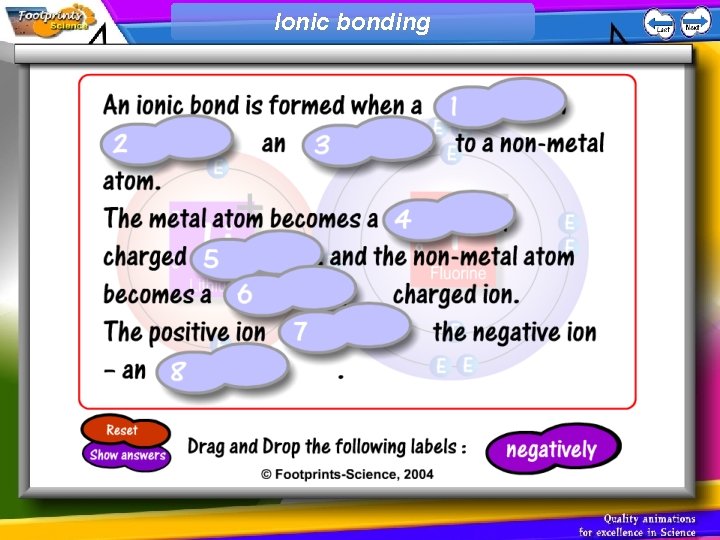
Ionic bonding

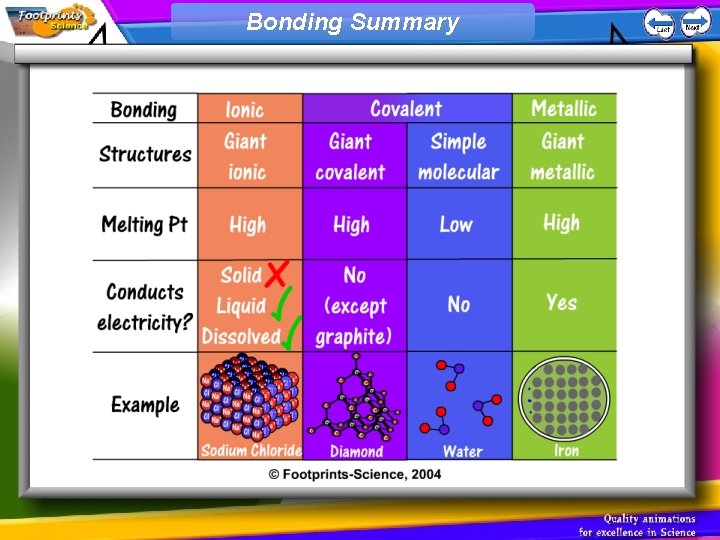
Ionic compounds 1. An ionic compound has a giant structure of ions (a giant ionic lattice) 2. The ionic bond is very strong – these substances have high melting and boiling points 3. Ionic compounds cannot conduct electricity when solid 4. When melted or dissolved in water the ions are free to move and the ionic compound can conduct electricity
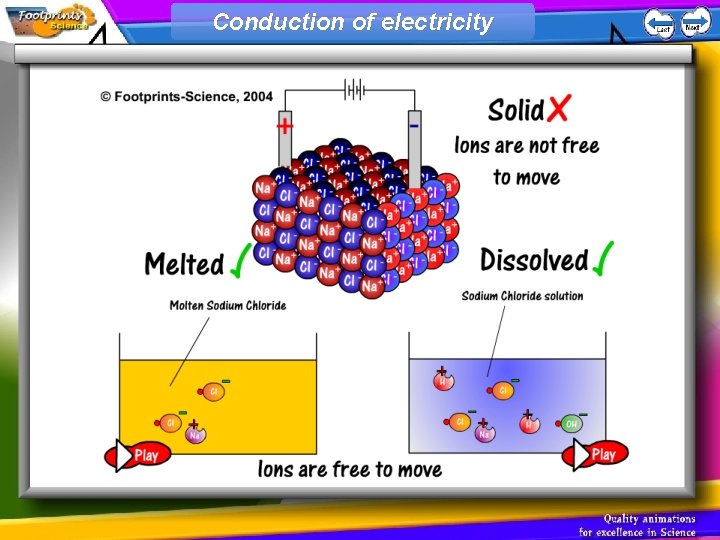
Conduction of electricity

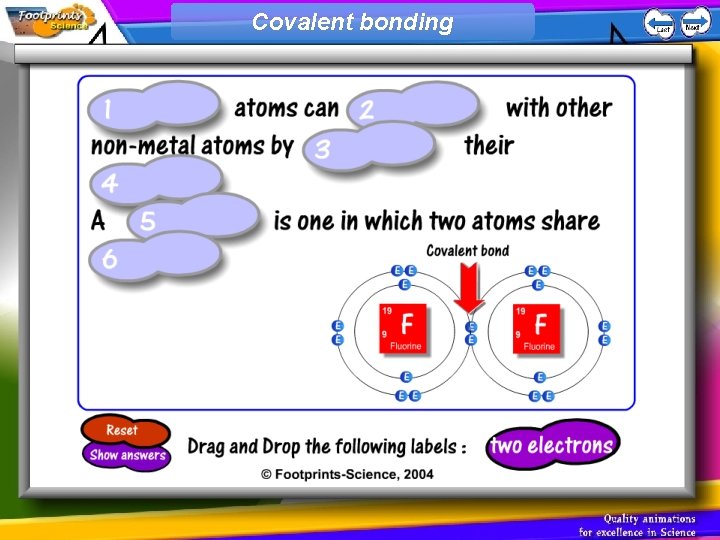
Covalent Bonding 1. Non-metal atoms can combine with other non-metal atoms by sharing their electrons 2. A covalent bond is one in which two atoms share two electrons
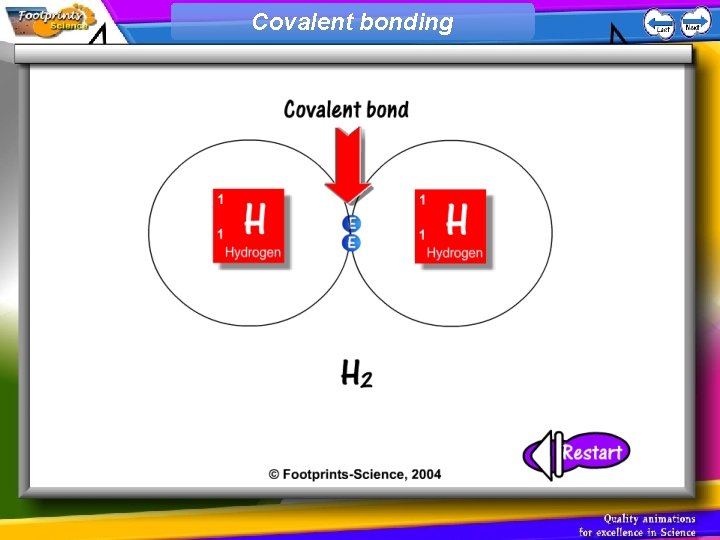
Covalent bonding
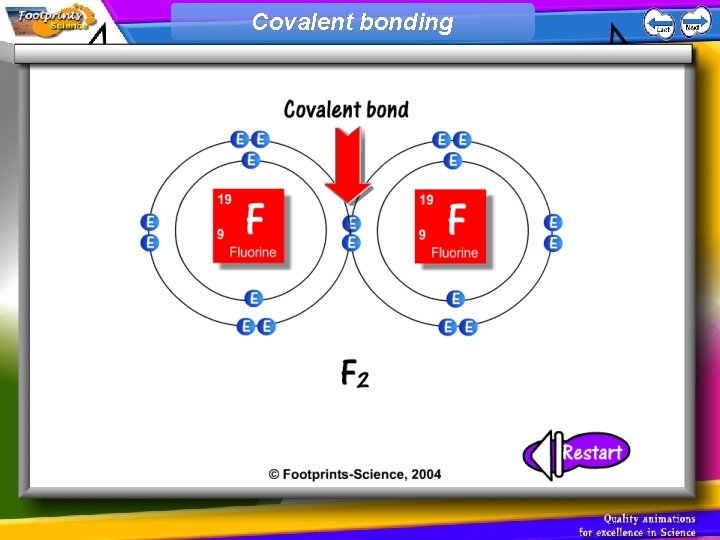
Covalent bonding
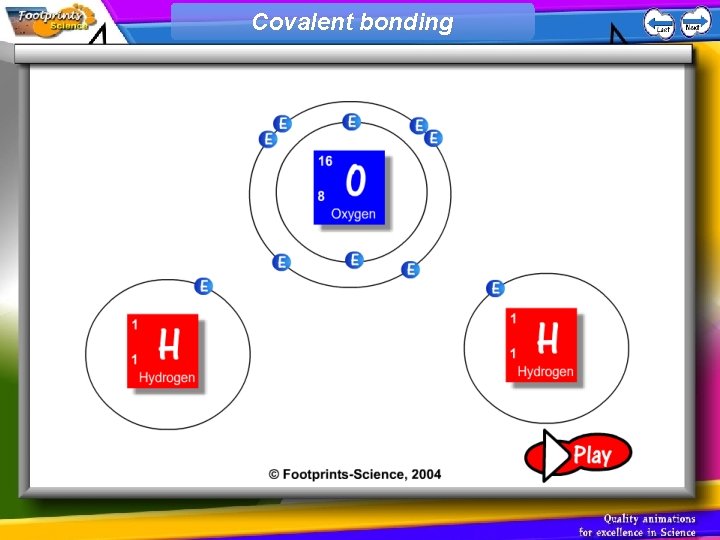
Covalent bonding
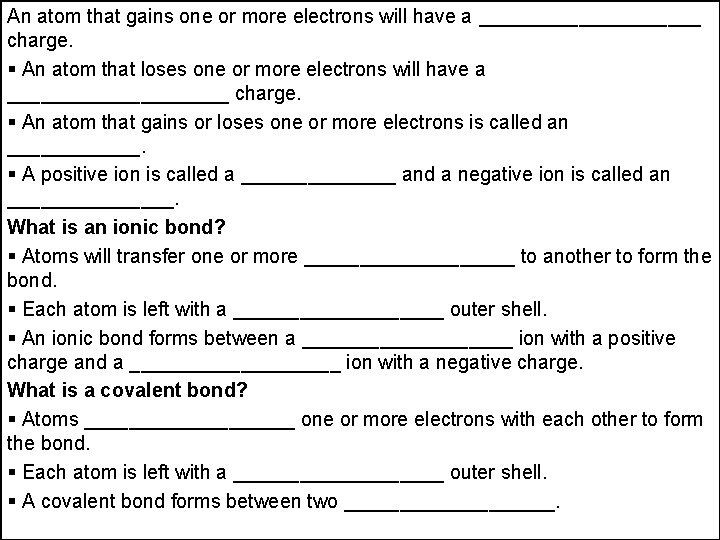
An atom that gains one or more electrons will have a __________ charge. An atom that loses one or more electrons will have a __________ charge. An atom that gains or loses one or more electrons is called an ______. A positive ion is called a _______ and a negative ion is called an ________. What is an ionic bond? Atoms will transfer one or more __________ to another to form the bond. Each atom is left with a __________ outer shell. An ionic bond forms between a __________ ion with a positive charge and a __________ ion with a negative charge. What is a covalent bond? Atoms __________ one or more electrons with each other to form the bond. Each atom is left with a __________ outer shell. A covalent bond forms between two __________.

Bonding Quiz • • Aluminum + Oxygen Magnesium + Oxygen Hydrogen + Fluorine Silicon + Hydrogen
Covalent bonding

Simple molecular compounds 1. Simple molecular compounds contain strong covalent bonds between atoms 2. However, there are weak forces between the molecules 3. Therefore, these compounds have low melting points and boiling points 4. The molecules do not carry an overall electric charge so these compounds do not conduct electricity

Simple molecular compounds

Giant covalent structures 1. Giant covalent structures contain strong covalent bonds between all atoms. Very high melting points. 2. In diamond, each carbon atom forms four covalent bonds 3. In graphite, each carbon atom forms three covalent bonds to form layers which can slide over each other. Free electrons in graphite allow it to conduct electricity.

Diamond
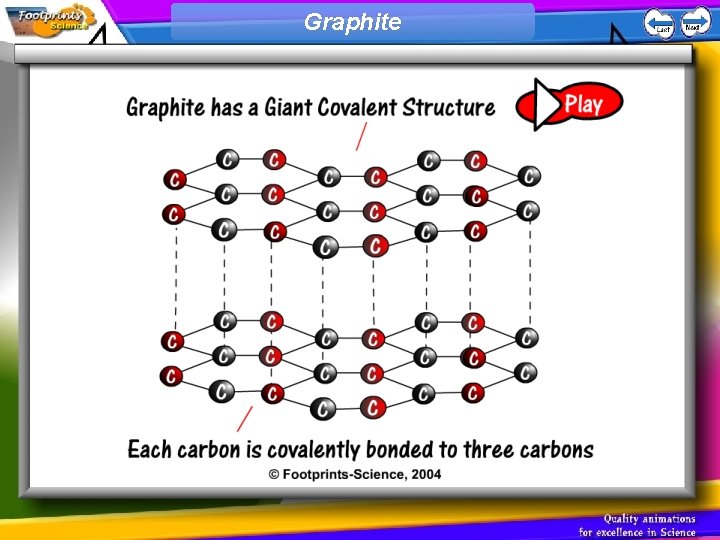
Graphite

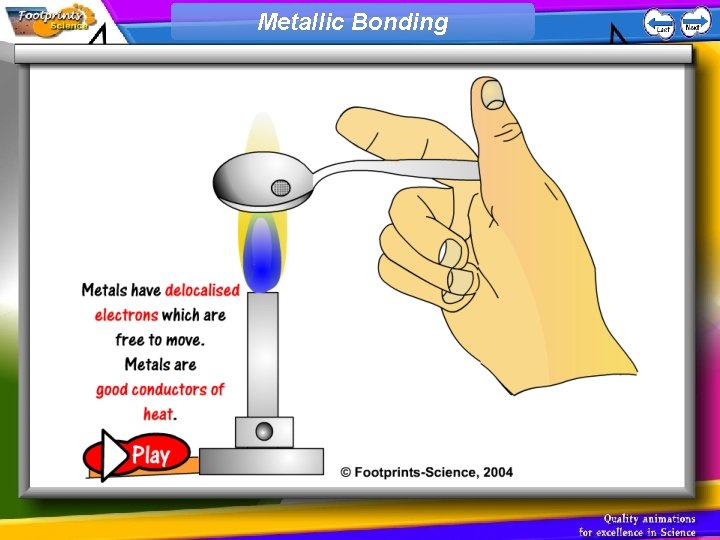
Metallic Bonding 1. Metals have giant structures 2. Metals have delocalised electrons which are free to move. These electrons; • allow the metal to conduct heat and electricity • hold the structure together so metals have very high melting points • allow the atoms to slide over each other so metals can be shaped
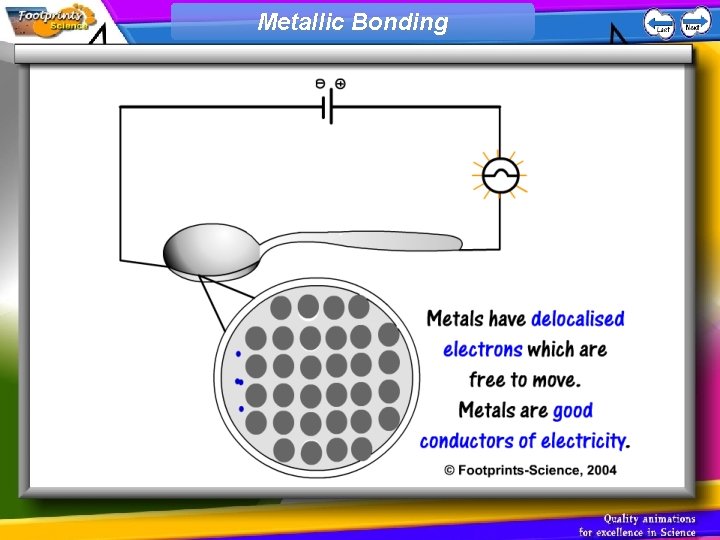
Metallic Bonding
Metallic Bonding
Bonding Summary
Wordsearch
Quiz
- Slides: 25