Bonding in Solids There are four classifications of
Bonding in Solids There are four classifications of solids, depending on the type of bonds that are present. • Ionic Solids • Covalent-Network Solids • Metallic Solids • Molecular Solids 1
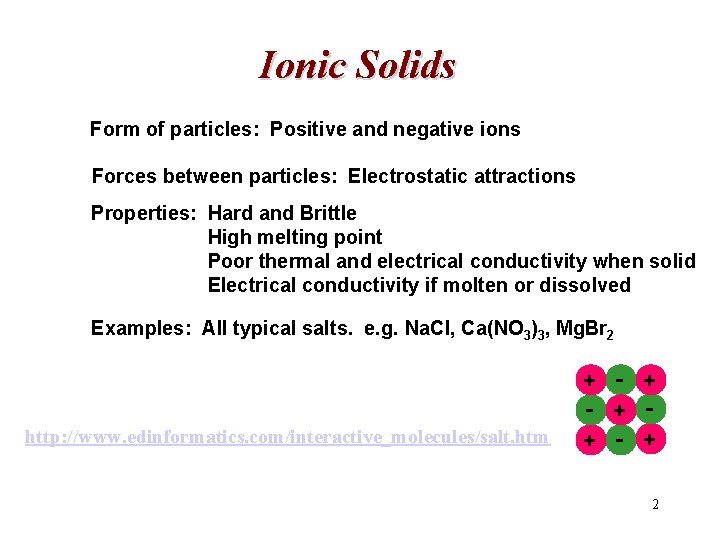
Ionic Solids Form of particles: Positive and negative ions Forces between particles: Electrostatic attractions Properties: Hard and Brittle High melting point Poor thermal and electrical conductivity when solid Electrical conductivity if molten or dissolved Examples: All typical salts. e. g. Na. Cl, Ca(NO 3)3, Mg. Br 2 - + + http: //www. edinformatics. com/interactive_molecules/salt. htm 2

Covalent-Network Solids Form of particles: Atoms connected in network of covalent bonds Forces between particles: Covalent bonds Properties: Very Hard Very high melting point Usually poor thermal and electrical conductivity Examples: Diamond (C), Quartz (Si. O 2), Silicon ( Si) Diamond Each carbon is connected to 4 others by a covalent bond http: //www. edinformatics. com/interactive_molecules/diamond. htm 3
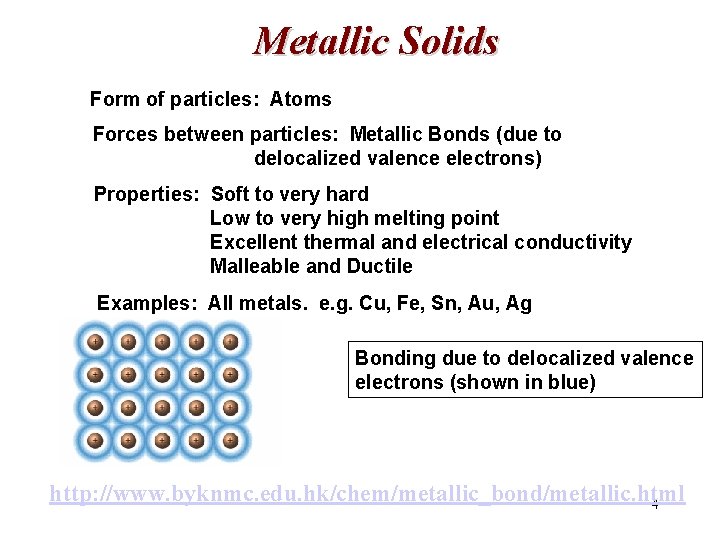
Metallic Solids Form of particles: Atoms Forces between particles: Metallic Bonds (due to delocalized valence electrons) Properties: Soft to very hard Low to very high melting point Excellent thermal and electrical conductivity Malleable and Ductile Examples: All metals. e. g. Cu, Fe, Sn, Au, Ag Bonding due to delocalized valence electrons (shown in blue) http: //www. byknmc. edu. hk/chem/metallic_bond/metallic. html 4
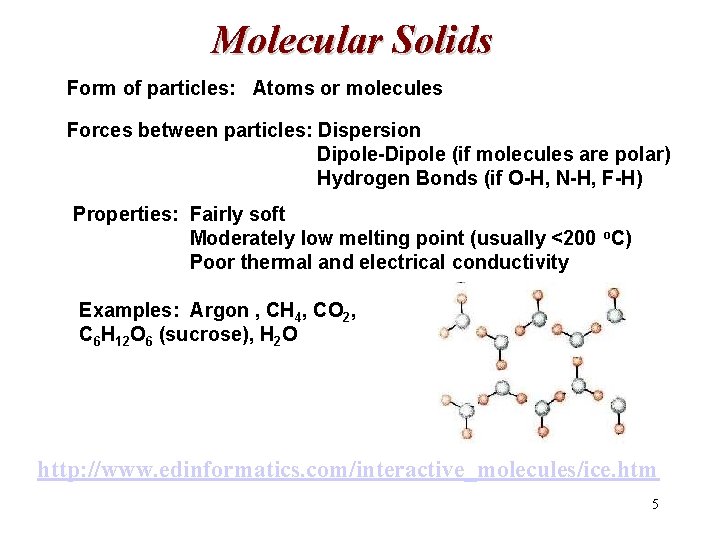
Molecular Solids Form of particles: Atoms or molecules Forces between particles: Dispersion Dipole-Dipole (if molecules are polar) Hydrogen Bonds (if O-H, N-H, F-H) Properties: Fairly soft Moderately low melting point (usually <200 o. C) Poor thermal and electrical conductivity Examples: Argon , CH 4, CO 2, C 6 H 12 O 6 (sucrose), H 2 O http: //www. edinformatics. com/interactive_molecules/ice. htm 5
- Slides: 5