Bonding in Metals Metallic Bonds Metallic bonds consist

Bonding in Metals

Metallic Bonds • Metallic bonds consist of the attraction of free -floating valence electrons and positively charged metal cations • The valence electrons are mobile and drift freely from one part of the metal to the other • The attraction between the valence electrons and the cations are what hold the bond together

7. 3 Metallic Properties • The sea of electrons explains the properties of metals • Metals are good conductors of electrical current because the electrons can flow freely in them
Metallic Properties • Metals are also ductiledrawn out into wire • Example- Copper wires
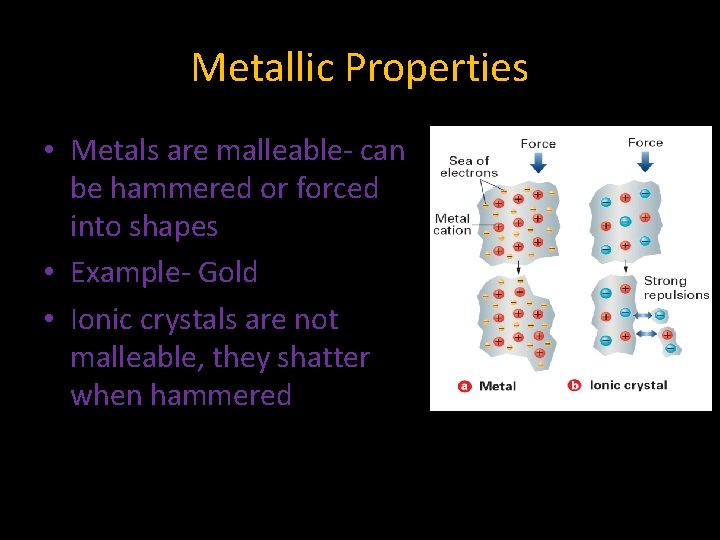
Metallic Properties • Metals are malleable- can be hammered or forced into shapes • Example- Gold • Ionic crystals are not malleable, they shatter when hammered

Metallic Properties • The valence electrons can explain why metals are ductile and malleable • When a metal is subjected to force the cations and electrons can slide past each other • When an ionic crystal is struck, the positive charges will repel and cause the crystal to shatter
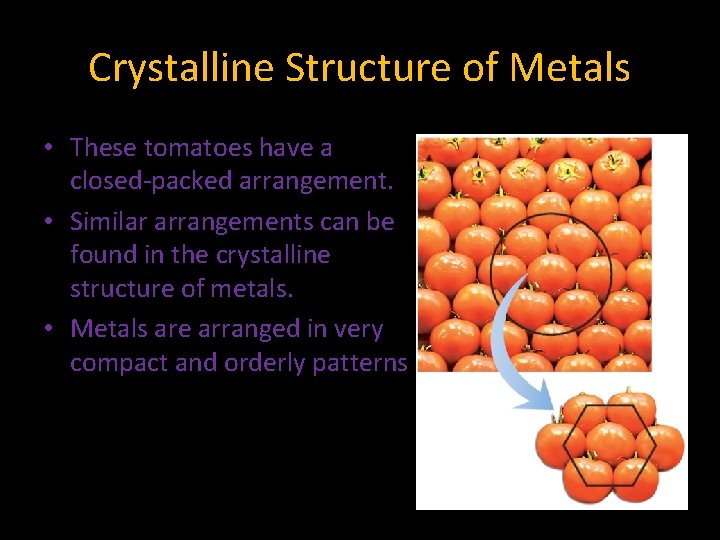
7. 3 Crystalline Structure of Metals • These tomatoes have a closed-packed arrangement. • Similar arrangements can be found in the crystalline structure of metals. • Metals are arranged in very compact and orderly patterns
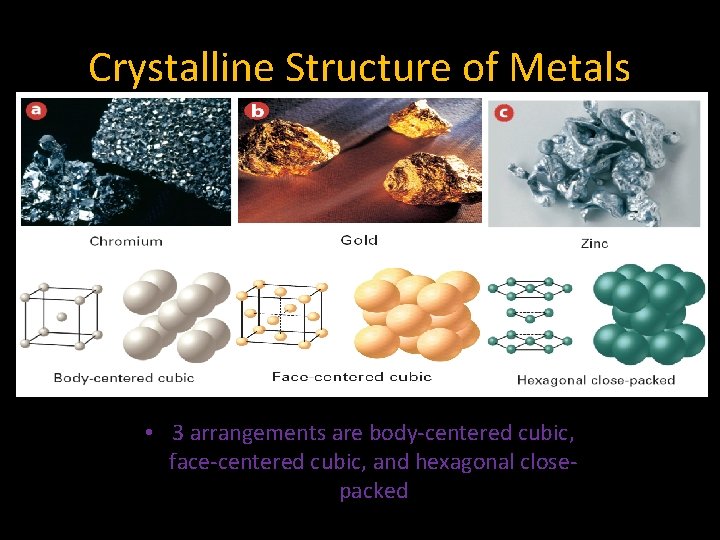
7. 3 Crystalline Structure of Metals • 3 arrangements are body-centered cubic, face-centered cubic, and hexagonal closepacked

7. 3 Alloys • Alloys are mixtures composed of two or more elements, at least one of which is a metal. • Alloys are important because their properties are often superior to those of their component elements. – Sterling Silver (Silver and Copper) – Bronze (Copper and Tin) – Aluminum Alloys (Aluminum and Manganese – Brass (Copper and Zinc)
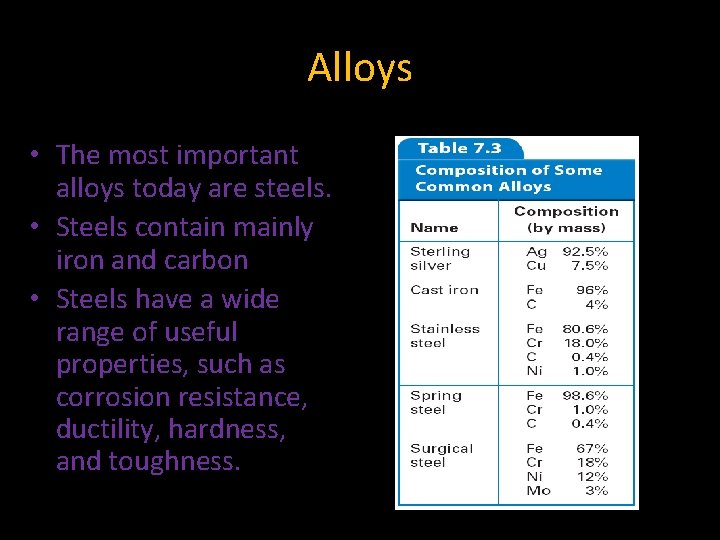
7. 3 Alloys • The most important alloys today are steels. • Steels contain mainly iron and carbon • Steels have a wide range of useful properties, such as corrosion resistance, ductility, hardness, and toughness.
- Slides: 10