Bonding in Cl Fn n1 7 Further Insights

Bonding in Cl. Fn (n=1 -7): Further Insights into Hypervalent Molecules and Recoupled Pair Bonds Lina Chen, David E. Woon and Thom H. Dunning Department of Chemistry University of Illinois at Urbana-Champaign Columbus, Ohio June 25, 2009
Motivation • Test the robustness of the recoupled pair bonding (RPB) model • Obtain the optimized structures and energies of low-lying states of Cl. Fn and Cl. F+ species • Explore the dependence of bond energies on the choice of the hypervalent atom, comparing S and Cl. • Identify factors that influence the bonding efficiency.
Methodology • RCCSD(T) : ground states of Cl. Fn (n=1 -7) and low-lying excited states for Cl. F and Cl. F 2 • MRCI/MRCI+Q: Cl. F (1 S+, 3 P), Cl. F+ (4 S-, 2 P) • GVB
Guidelines 1 S+ Cl F 90 Cl
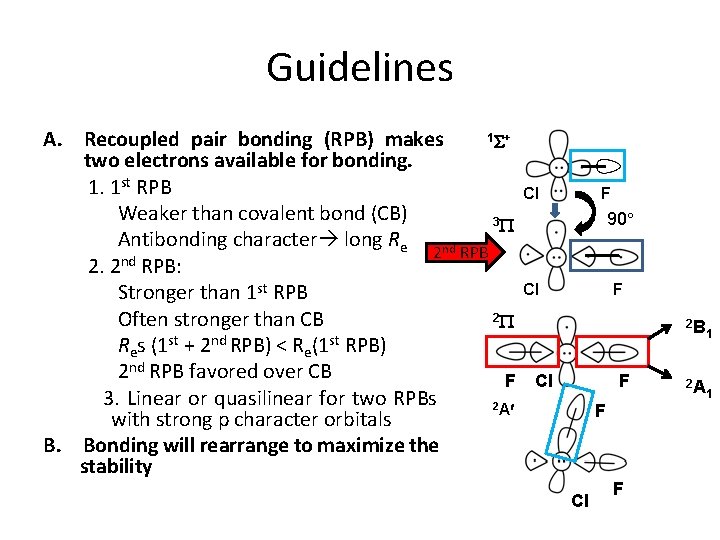
Guidelines 1 S+ A. Recoupled pair bonding (RPB) makes two electrons available for bonding. 1. 1 st RPB Weaker than covalent bond (CB) 3 P Antibonding character long Re 2 nd RPB nd 2. 2 RPB: Stronger than 1 st RPB 2 P Often stronger than CB Res (1 st + 2 nd RPB) < Re(1 st RPB) 2 nd RPB favored over CB F 3. Linear or quasilinear for two RPBs 2 A with strong p character orbitals B. Bonding will rearrange to maximize the stability F Cl 90 Cl F F Cl F 2 B 1 2 A 1
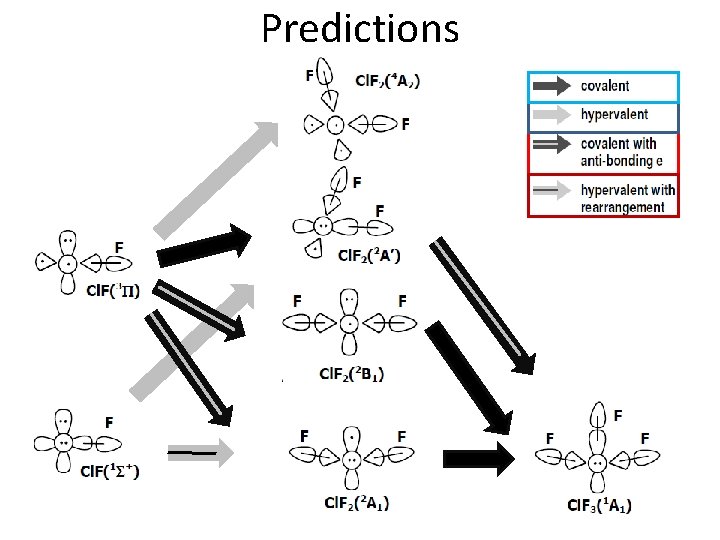
Predictions
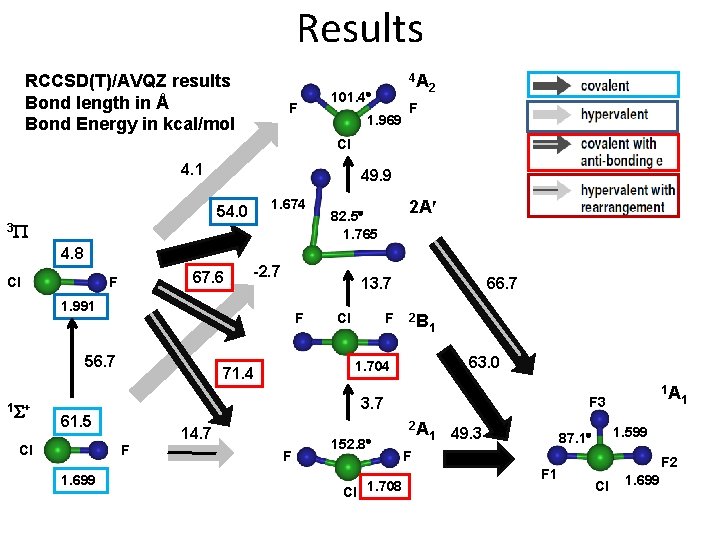
Results RCCSD(T)/AVQZ results Bond length in Å Bond Energy in kcal/mol F 4 A 101. 4 1. 969 2 F Cl 4. 1 49. 9 1. 674 54. 0 3 P 2 A 82. 5 1. 765 4. 8 Cl 67. 6 F -2. 7 13. 7 1. 991 F 56. 7 1 S+ Cl F 66. 7 2 B 1 63. 0 1. 704 71. 4 3. 7 61. 5 Cl 14. 7 F 1. 699 F 152. 8 Cl 1. 708 1 A F 3 2 A 1 49. 3 1. 599 87. 1 F F 1 F 2 Cl 1. 699 1
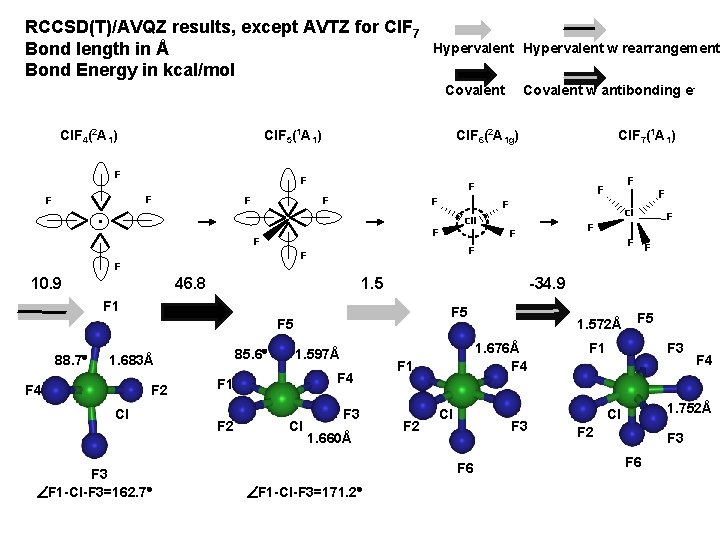
RCCSD(T)/AVQZ results, except AVTZ for Cl. F 7 Bond length in Å Bond Energy in kcal/mol Hypervalent w rearrangement Covalent Cl. F 4(2 A 1) Cl. F 5(1 A 1) F Cl. F 6(2 A 1 g) F F Covalent w antibonding e. Cl. F 7(1 A 1) F F F 10. 9 1. 5 F 5 85. 6 1. 683Å F 4 F 2 Cl F 3 F 1 -Cl-F 3=162. 7 1. 597Å F 4 F 1 F 2 Cl F 3 1. 660Å F 1 F 2 1. 572Å 1. 676Å F 4 Cl F 3 F 6 F 1 -Cl-F 3=171. 2 F -34. 9 F 1 88. 7 F F 46. 8 F F F Cl Cll F F 5 F 1 F 3 F 4 1. 752Å Cl F 2 F 3 F 6
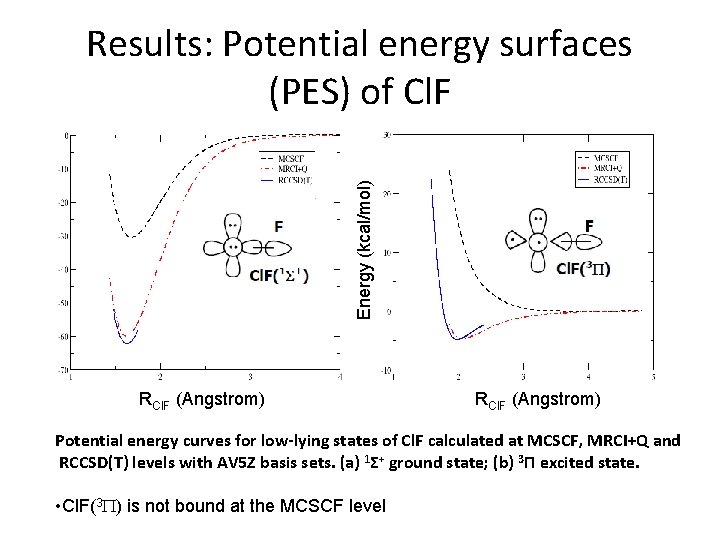
Energy (kcal/mol) Results: Potential energy surfaces (PES) of Cl. F RCl. F (Angstrom) Potential energy curves for low-lying states of Cl. F calculated at MCSCF, MRCI+Q and RCCSD(T) levels with AV 5 Z basis sets. (a) 1Σ+ ground state; (b) 3 П excited state. • Cl. F(3 P) is not bound at the MCSCF level
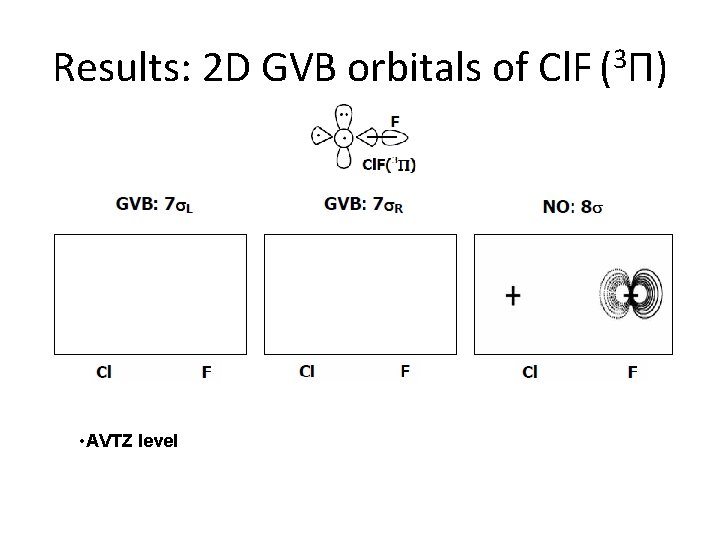
Results: 2 D GVB orbitals of Cl. F (3 П) • AVTZ level
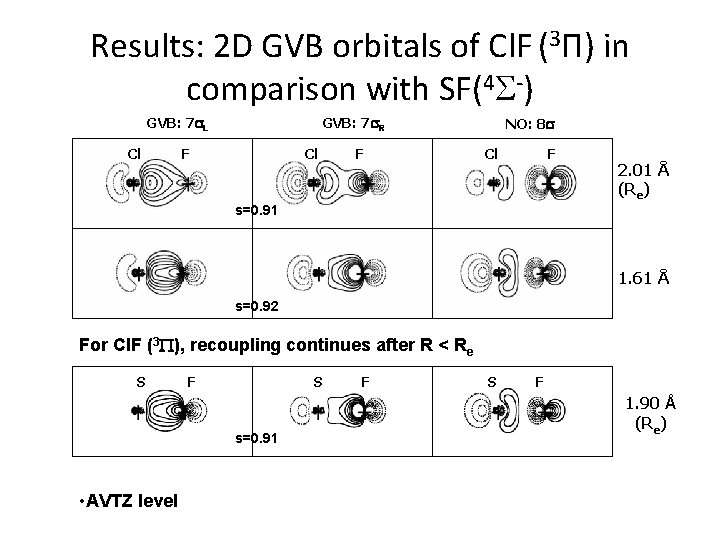
Results: 2 D GVB orbitals of Cl. F (3 П) in comparison with SF(4 S-) GVB: 7 s. L Cl GVB: 7 s. R F Cl F NO: 8 s Cl F 2. 01 Å (Re) s=0. 91 1. 61 Å s=0. 92 For Cl. F (3 P), recoupling continues after R < Re S F S s=0. 91 • AVTZ level F S F 1. 90 Å (Re)
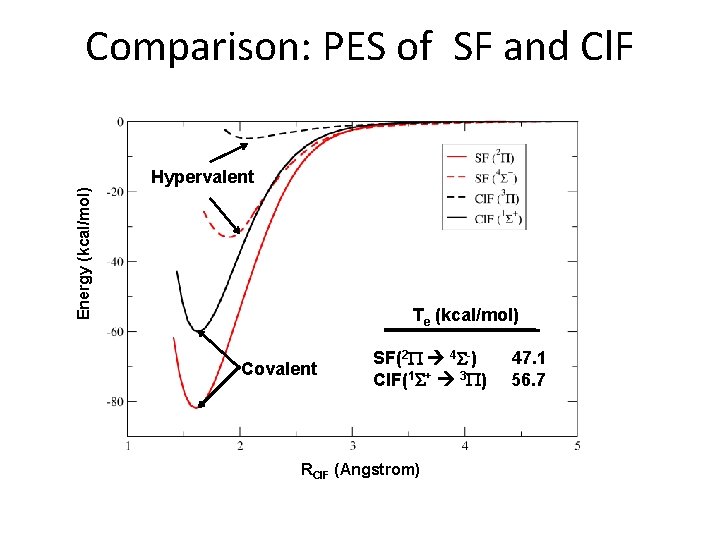
Comparison: PES of SF and Cl. F Energy (kcal/mol) Hypervalent Te (kcal/mol) Covalent SF(2 P 4 S-) Cl. F(1 S+ 3 P) RCl. F (Angstrom) 47. 1 56. 7
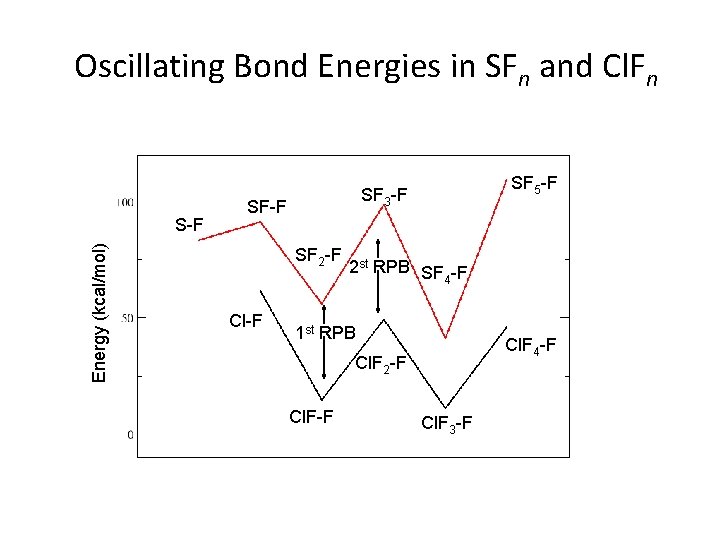
Oscillating Bond Energies in SFn and Cl. Fn Energy (kcal/mol) S-F SF 2 -F Cl-F SF 5 -F SF 3 -F 2 st RPB SF -F 4 1 st RPB Cl. F 4 -F Cl. F 2 -F Cl. F 3 -F
Conclusions • Trends in the calculated geometries and energies agree with predictions using recoupled pair bonding model. • Similar oscillating trends are found in both the SFn and Cl. Fn series, and the differences are consistent with the difference between S and Cl at the atomic level. • Future work: a. PFn series as well as various combinations of P, S, Cl and F with other ligands such as monovalent H, Cl, and OH and divalent O. b. Reactions: Cl. F+F 2 Cl. F 3

Acknowledgment • Funded by the Distinguished Chair for Research Excellence in Chemistry at the University of Illinois at Urbana-Champaign.
- Slides: 15