Bonding Electronegativity Bond Type 2 bond types Covalent
Bonding

Electronegativity & Bond Type • 2 bond types: – Covalent – Ionic

Electronegativity • Ability of an atom to attract electrons in a bond • Higher electronegativity = more pull on electrons


Tug of war • H vs H • H vs F
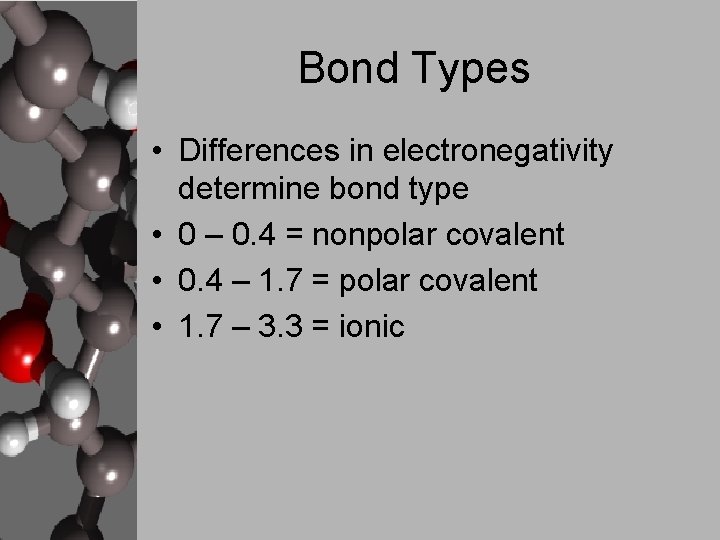
Bond Types • Differences in electronegativity determine bond type • 0 – 0. 4 = nonpolar covalent • 0. 4 – 1. 7 = polar covalent • 1. 7 – 3. 3 = ionic

Nonpolar Covalent 0 – 0. 4 • Electrons equally shared – Directly in the middle of the two atoms • H 2 • O 2

Polar Covalent 0. 4 – 1. 7 • Electrons are shared by not equally – More electronegative atom pulls electrons closer • HCl • CCl 4

Ionic >1. 7 • Electrons not shared at all – Atom with higher electronegativity takes electrons from atom with lower electronegativity • Na. Cl • K 2 O
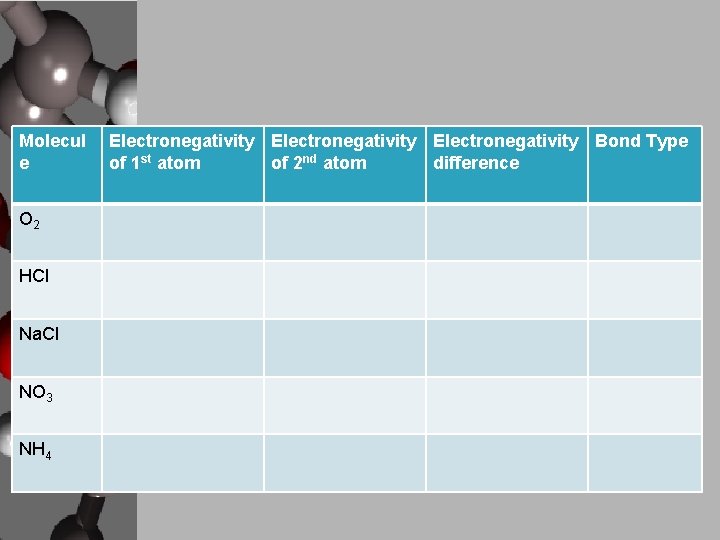
Molecul e O 2 HCl Na. Cl NO 3 NH 4 Electronegativity Bond Type of 1 st atom of 2 nd atom difference

Ion Formation • Ions are formed when atoms gain or lose valence electrons to achieve a stable octet electron configuration

Valence Electrons and Chemical Bonds • Recall: – All elements in the same group have the same number of valence electrons and therefore…

• Valence electrons are involved in the formation of chemical bonds – The force that holds two atoms together – Attraction between positive and negative ions

• Recall: Dot structures – Show only valence electrons – Carbon: 1 s 2 2 p 2 – Bromine: [Ar] 4 s 2 3 d 10 4 p 5

• Recall: octet rule – Atoms will gain, lose, or share electrons to acquire the stable electron configuration of a noble gas – Metals – – Nonmetals -

Positive Ion formation • Name: • Formed by: • Group 1: • Group 2: • Group 13:

• Transition metal ions • Form cations only • Charges may vary in some atoms – Fe can lose 2 or 3 electrons – Fe 2+ or Fe 3+ – Periodic table

Negative Ion Formation • Name: • Formed by: • Group 15 • Group 16 • Group 17
Ionic Bonds & Ionic Compounds • Oppositely charged ions attract each other, forming electrically neutral compounds

Formation of an Ionic Bond • Ionic bond – the force of attraction that holds oppositely charged ions together

• Ionic compounds – compounds that contain ionic bonds – Cation + anion – Metal + nonmetal – Also called salts
• Binary ionic compounds – contain two different elements (a metal + a nonmetal) – Na. Cl – Mg. O – K 2 S – Ca. I 2
• Compound formation and charge – Ionic compounds are electrically neutral – Total positive charge must = total negative charge – Net charge of all ionic compounds = 0
![Formation of Sodium Chloride • Na: [Ne]3 s 1 + Cl: [Ne]3 s 23 Formation of Sodium Chloride • Na: [Ne]3 s 1 + Cl: [Ne]3 s 23](http://slidetodoc.com/presentation_image_h2/8ddf5f38b61dc664411341a640e24171/image-24.jpg)
Formation of Sodium Chloride • Na: [Ne]3 s 1 + Cl: [Ne]3 s 23 p 5 • Dot Structure: Na + Cl [Na]+ +[ Cl ]-
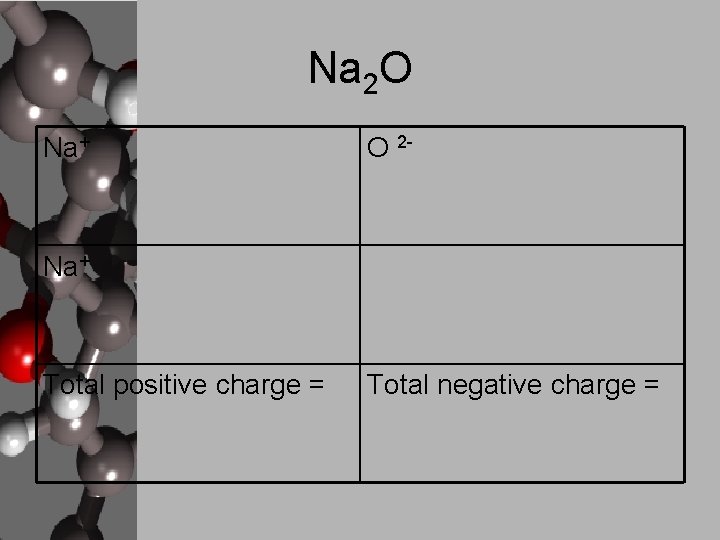
Na 2 O Na+ O 2 - Na+ Total positive charge = Total negative charge =

Al 2 O 3 Al 3+ O 2 - Al 3+ O 2 O 2 - Total positive charge = Total negative charge =

How would an ionic compound form from each of the following: • Na + N • Li + O • Sr + F • Group 1 + group 15

Formulas for ionic compounds • Formula unit = the chemical formula for an ionic compound – Simplest ratio of ions involved – Mg 6 Cl 12 – Mg. Cl 2 – Overall charge = 0

• Monatomic ions – one atom ions – Ex: • Oxidation number - the charge of a monatomic ion – Most transition metals have more than one oxidation number

What is the oxidation number of the ions in the following compounds? • Fe. O • Mg. Cl 2 • Cu 3 N 2 • Fe 2 O 3
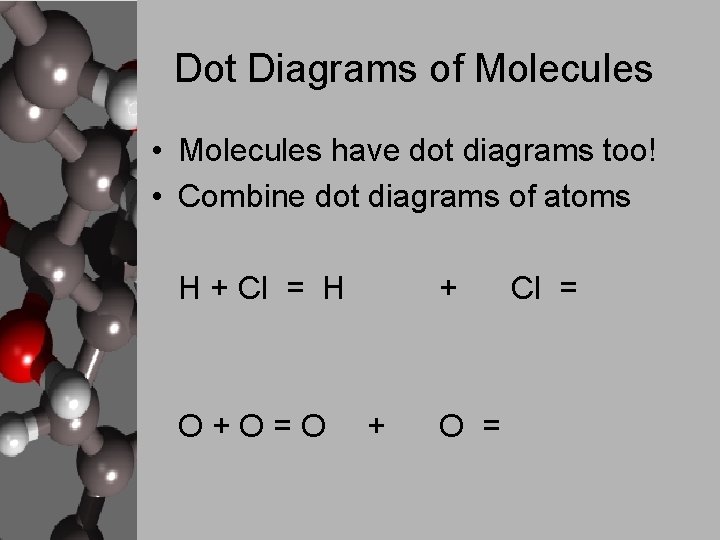
Dot Diagrams of Molecules • Molecules have dot diagrams too! • Combine dot diagrams of atoms H + Cl = H O+O=O + + O = Cl =
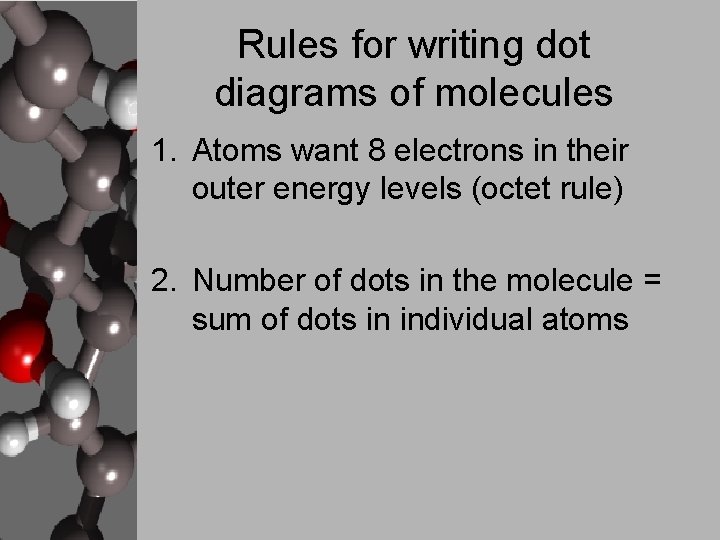
Rules for writing dot diagrams of molecules 1. Atoms want 8 electrons in their outer energy levels (octet rule) 2. Number of dots in the molecule = sum of dots in individual atoms
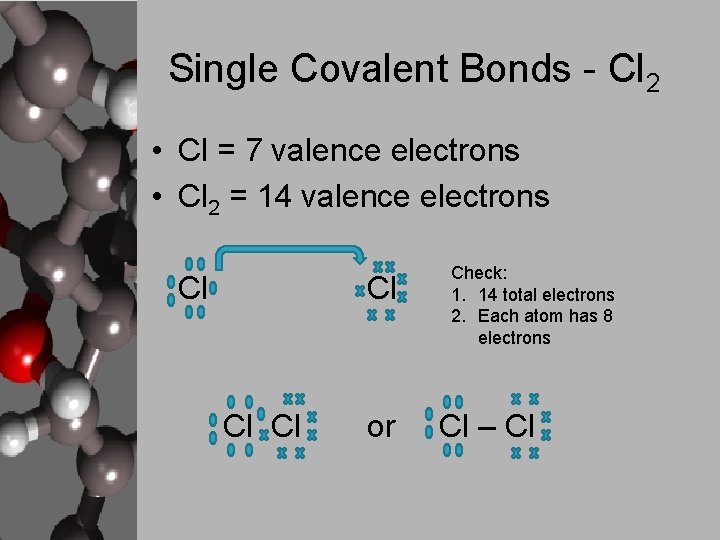
Single Covalent Bonds - Cl 2 • Cl = 7 valence electrons • Cl 2 = 14 valence electrons Cl Cl or Check: 1. 14 total electrons 2. Each atom has 8 electrons Cl – Cl
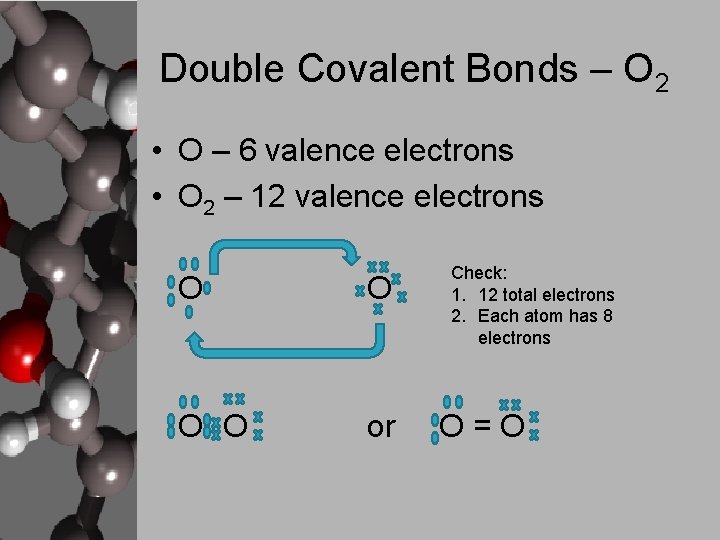
Double Covalent Bonds – O 2 • O – 6 valence electrons • O 2 – 12 valence electrons O O or Check: 1. 12 total electrons 2. Each atom has 8 electrons O=O
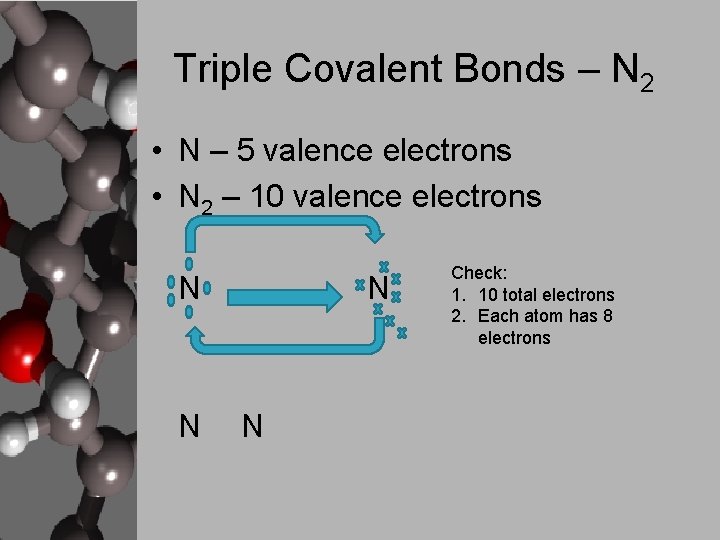
Triple Covalent Bonds – N 2 • N – 5 valence electrons • N 2 – 10 valence electrons N N Check: 1. 10 total electrons 2. Each atom has 8 electrons

Rules of Covalent Bonding • If atoms need 1 electron, it will usually form 1 covalent bond. • If atoms need 2 electrons, it will usually form 2 covalent bonds. • If atoms needs 3 electrons, it will usually form 3 covalent bonds.
Examples • CH 4 • NH 3 • CO 2
Challenges! • SO 3 • PO 43 • SO 42 -

Polyatomic Ions • A group of two or more atoms that act as one ion and have one charge • ALWAYS STAY TOGETHER IN REACTIONS
Dot Diagrams of Polyatomic Ions • Number of dots in the molecule must take the ions charge into account • NH 41+ –N– 5 – H – 1(4) – +1 charge – subtract 1 electron
• PO 43–P– 5 – O – 6(4) – -3 charge – add 3 electrons
Molecular Geometry • Molecules have specific shapes • You already know a lot of the shapes from doing the dot diagrams!
VSEPR Theory • • • V alence S hell E lectron P air R epulsion • The shape of a molecule is determined by minimizing repulsion between lone pairs
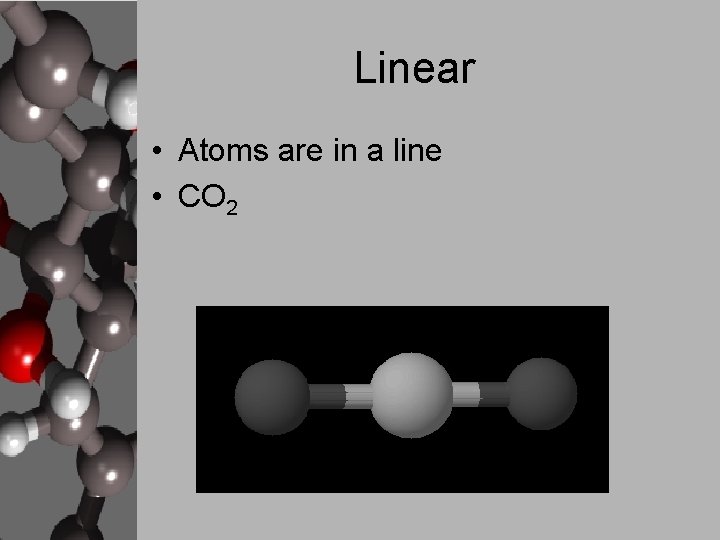
Linear • Atoms are in a line • CO 2
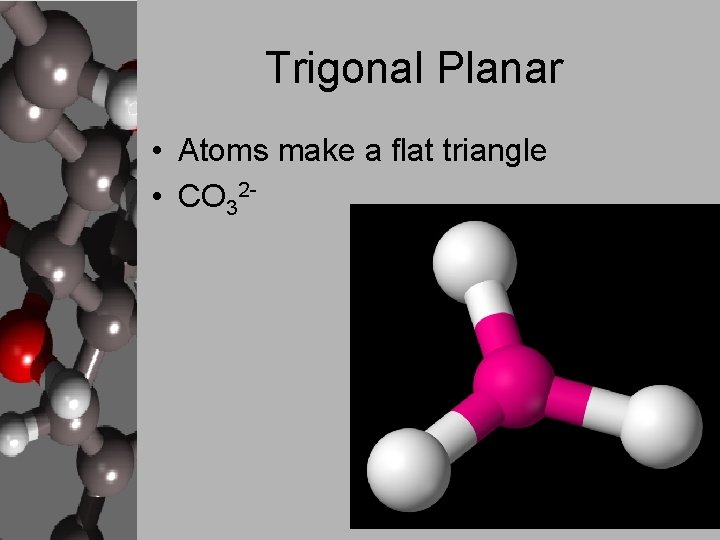
Trigonal Planar • Atoms make a flat triangle • CO 32 -
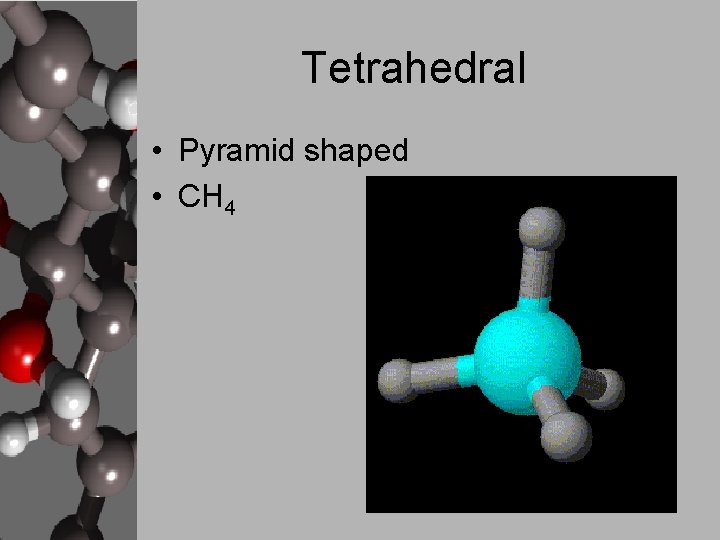
Tetrahedral • Pyramid shaped • CH 4
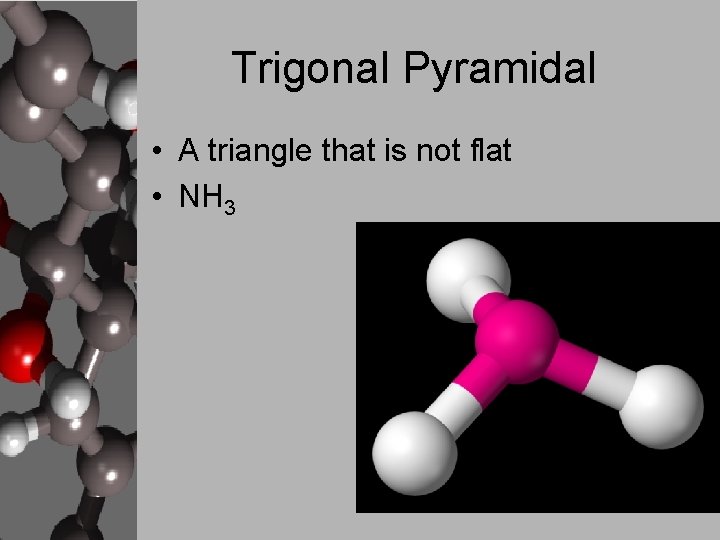
Trigonal Pyramidal • A triangle that is not flat • NH 3
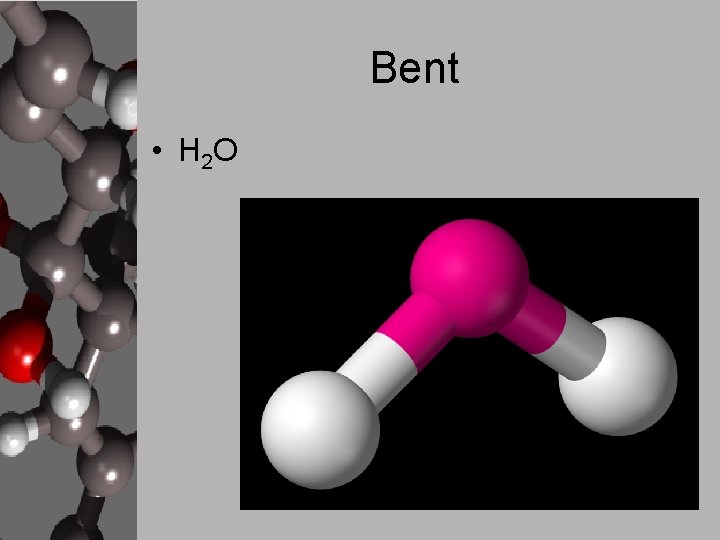
Bent • H 2 O
Polar and Nonpolar Molecules • Nonpolar bond = electrons shared equally • Polar bond = electrons shared unequally (dipole) – More electronegative atom pulls electrons closer to it and therefore has a slightly negative charge – Less electronegative atom has a slightly positive charge

Polar molecules always have: 1. At least one polar covalent bond 2. Asymmetric geometry 1. Lone pairs on the center atom 2. Different atoms bonded to the center atom
• HCl • H 2 O

• Not every molecule with polar bonds is polar!!! • CH 4 • CO 2
Polar Bond vs. Polar Molecule • Polar bond = electrons shared unequally between molecules • Polar molecule = entire molecule has different partial charges on opposite sides of the molecule • Depends on shape
• CCl 4 • NH 3 • H 20 – Linear vs. bent

Intermolecular Forces • Sometimes polar molecules can attract each other • Weaker than bonds

Dispersion forces • WEAKEST!!! • Caused by attraction that results from temporary closeness of electrons • Only last a fraction of a second

Dipole – Dipole forces • Occur when polar molecules are attracted to one another • Permanent attraction

Hydrogen Bonds • STRONGEST!!! • Between H of one polar molecule and O, N, or F of another polar molecule • Without them we wouldn’t exist
- Slides: 58