Bonding and structure What is an ionic bond
Bonding and structure What is an ionic bond ?
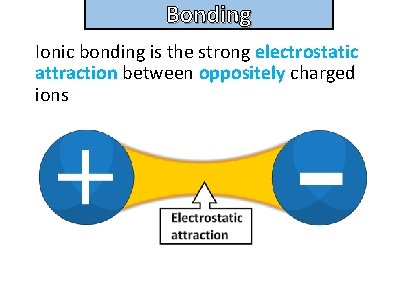
Bonding and structure Ionic bonding is the strong electrostatic attraction between oppositely charged ions

Bonding and structure describe the effects that ionic radius and ionic charge have on the strength of ionic bonding

Bonding and structure Ionic charge • The greater the charge on an ion the stronger the ionic bond Ionic radius • The smaller distance between the ions the stronger the ionic bond. • Ions with a smaller radius can pack closer together, thereby increasing the strength of ionic bonding

Bonding and structure describe the formation of ions in terms of electron loss or gain

Bonding and structure A positive ion (cation) is formed what an atom looses and electrons A negative (anion) ion is formed when an atom gains electrons
Bonding and structure draw the electronic configuration diagrams of Sodium (cation) and chlorine (anion ) using dot-and-cross diagrams

Bonding and structure

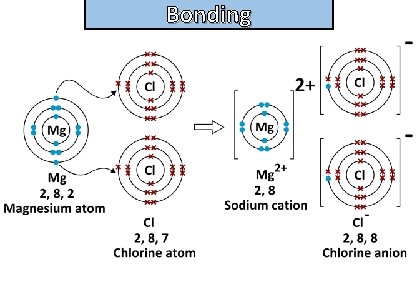
Bonding and structure draw the electronic configuration diagrams of magnesium (cation) and chlorine (anion) using dot-and-cross diagrams
Bonding

Bonding and structure explain the trends in ionic radii down a group
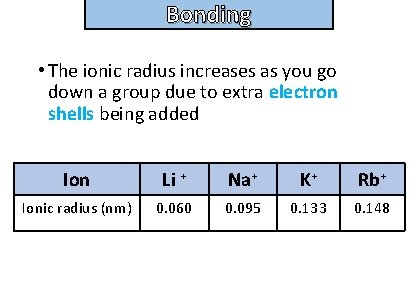
Bonding and structure • The ionic radius increases as you go down a group due to extra electron shells being added Ion Li + Na+ K+ Rb+ Ionic radius (nm) 0. 060 0. 095 0. 133 0. 148
Bonding and structure Define what an isoelectric ion is

Bonding and structure • Isoelectronic ions are ions of different atoms with the same number of electrons. • Example: N 3 - has 10 electrons and O 2 - also has 10 electrons

Bonding and structure explain the trends in ionic radii for a set of isoelectronic ions, e. g. N 3 - to Al 3+
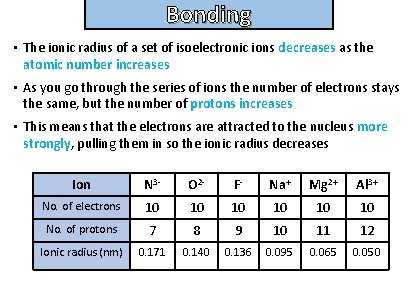
Bonding and structure • The ionic radius of a set of isoelectronic ions decreases as the atomic number increases • As you go through the series of ions the number of electrons stays the same, but the number of protons increases • This means that the electrons are attracted to the nucleus more strongly, pulling them in so the ionic radius decreases Ion N 3 - O 2 - F- Na+ Mg 2+ Al 3+ No. of electrons 10 10 10 No. of protons 7 8 9 10 11 12 Ionic radius (nm) 0. 171 0. 140 0. 136 0. 095 0. 065 0. 050

Bonding and structure Describe how the physical properties of ionic compounds provide evidence for the existence of ions

Bonding and structure 1. High melting points Oppositely charged ions would be attracted by strong electrostatic forces which would be difficult to overcome 2. Soluble in water but not in non-polar solvents Charged ions would be pulled apart by water but not by non-polar solvents 3. Ionic compounds don’t conduct electricity when solid but do when molten or dissolved Ions would only be able to carry a charge when free to move 4. Ionic compounds are brittle Oppositely charged ions are held together in a lattice structure. When you distort the shape of the lattice you move sections of the lattice over parts of the lattice with ions of the same charge. When this happens the ions in the compound undergo electrostatic repulsion, splitting the compound.

Bonding and structure Describe how the migration of ions provide evidence for the existence of ions
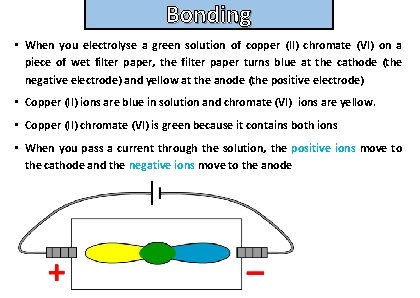
Bonding and structure • When you electrolyse a green solution of copper (II) chromate (VI) on a piece of wet filter paper, the filter paper turns blue at the cathode (the negative electrode) and yellow at the anode (the positive electrode) • Copper (II) ions are blue in solution and chromate (VI) ions are yellow. • Copper (II) chromate (VI) is green because it contains both ions • When you pass a current through the solution, the positive ions move to the cathode and the negative ions move to the anode

Describe how electron density maps for ionic compounds provide evidence for the existence of ions in ionic compounds

In electron density maps for ionic compounds, there is no single line representing electron density that surrounds both cations and anions

Draw an electron density map for Na. Cl
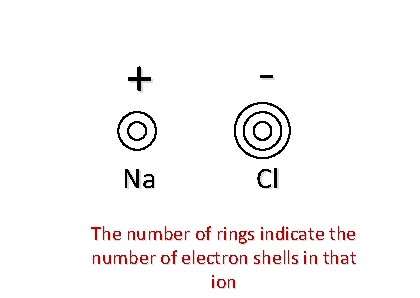
+ - Na Cl The number of rings indicate the number of electron shells in that ion
Bonding and structure What is a covalent bond ?

Bonding and structure A covalent bond is the • strong electrostatic attraction • between two nuclei • and the shared pair of electrons between them
Bonding and structure draw a dot-and-cross diagram to show the electron arrangement in a molecule of chlorine cl 2
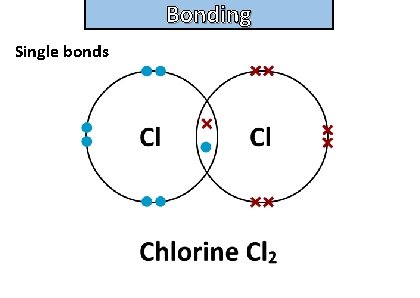
Bonding and structure Single bonds

Bonding and structure draw a dot-and-cross diagram to show the electron arrangement in a molecule of hydrogen chloride HCl
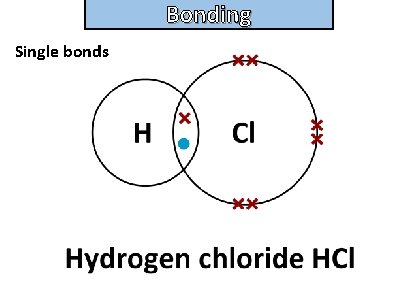
Bonding and structure Single bonds
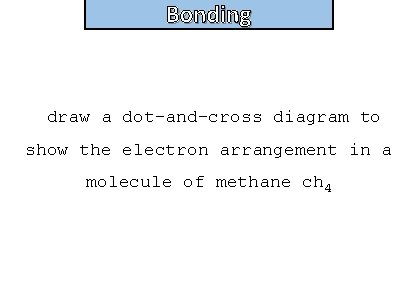
Bonding and structure draw a dot-and-cross diagram to show the electron arrangement in a molecule of methane ch 4
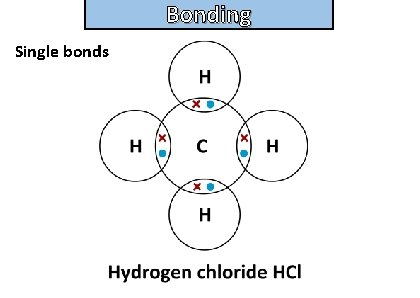
Bonding and structure Single bonds

Bonding and structure draw a dot-and-cross diagram to show the electron arrangement in a molecule of water H 2 O

Bonding and structure Single bonds

Bonding and structure draw a dot-and-cross diagram to show the electron arrangement in a molecule of oxygen o 2
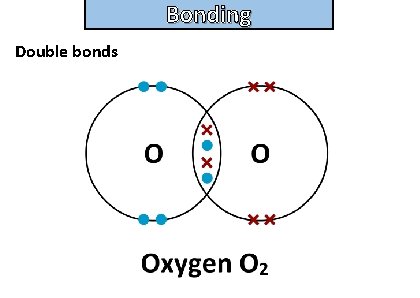
Bonding and structure Double bonds

Bonding and structure draw a dot-and-cross diagram to show the electron arrangement in a molecule of nitrogen N 2

Bonding and structure Triple bonds
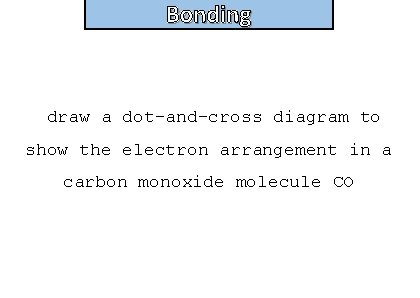
Bonding and structure draw a dot-and-cross diagram to show the electron arrangement in a carbon monoxide molecule CO
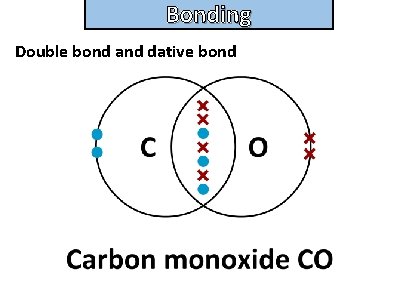
Bonding and structure Double bond and dative bond

Bonding and structure draw a dot-and-cross diagram to show the electron arrangement in an aluminum chloride molecule
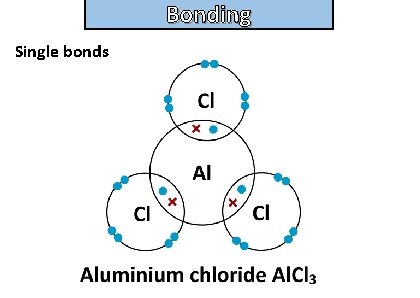
Bonding and structure Single bonds

Bonding and structure draw a dot-and-cross diagram to show the electron arrangement in a molecule of Aluminum chloride Al 2 Cl 6
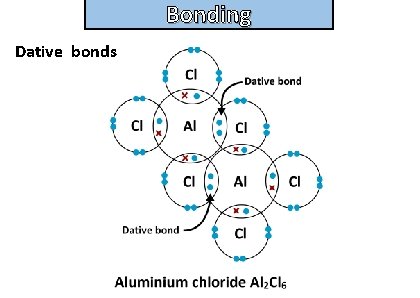
Bonding and structure Dative bonds
Bonding and structure what is dative (coordinate) bonding ?
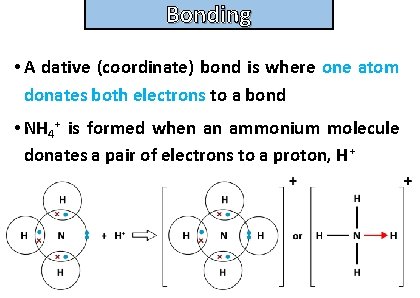
Bonding and structure • A dative (coordinate) bond is where one atom donates both electrons to a bond • NH 4+ is formed when an ammonium molecule donates a pair of electrons to a proton, H+
Bonding and structure Explain the relationship between bond lengths and bond strengths for covalent bond
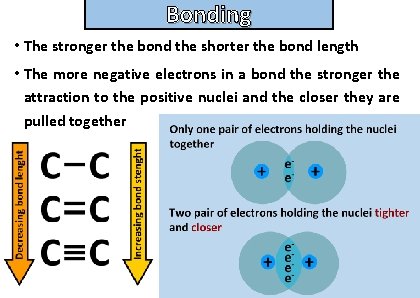
Bonding and structure • The stronger the bond the shorter the bond length • The more negative electrons in a bond the stronger the attraction to the positive nuclei and the closer they are pulled together

Bonding and structure Describe how the shape of a simple molecule or ion is determined by the repulsion between the electron pairs that surround a central atom

Bonding and structure • Electrons are negatively charged so will repel each other as much as possible • The shape of a simple molecule or ion depends on the number of pairs of electrons and type of pair in the outer shell of the central atom • Lone pairs repel more than bonding pairs

Bonding and structure List the steps involved in predicting the shape of a molecule according to electron-pair repulsion theory

Bonding and structure 1. Determine which atom is the central atom 2. Work out the number of electrons in the outer shell of the central atom using the periodic table 3. Use the molecular formula to find the number of atoms bonded to the central atom and then determine the number of shared electrons 4. Add up the number of shared electrons and divide by two to find the number of electron pairs. If you have an ion you must account for its charge 5. Compare the number of electron pairs with the number of bonds to find the number of lone pairs 6. Given the number of lone pairs and bonding pairs determine the molecule shape

Bonding and structure For the following molecules state the number of electron pairs around the central atom, the number of lone pairs and their shape type. Then draw the molecule: • Be. Cl 2 • CO 2

Bonding and structure 2 electron pairs around the central atom Formula : Be. Cl 2 Number of lone pairs : 0 Shape type: linear Formula : CO 2 Number of lone pairs : 0 Shape type: linear

Bonding and structure For the following molecules state the number of electron pairs around the central atom, the number of lone pairs and their shape type. Then draw the molecule: • BCl 3 • s. O 2
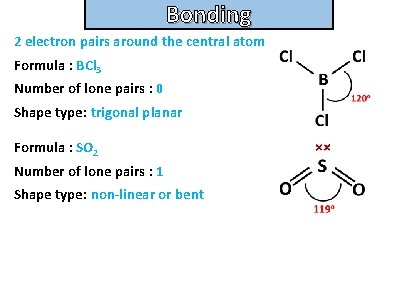
Bonding and structure 2 electron pairs around the central atom Formula : BCl 3 Number of lone pairs : 0 Shape type: trigonal planar Formula : SO 2 Number of lone pairs : 1 Shape type: non-linear or bent
Bonding and structure For the following molecules state the number of electron pairs around the central atom, the number of lone pairs and their shape type. Then draw the molecule: • Nh 4+ • Pf 3 • H 2 O
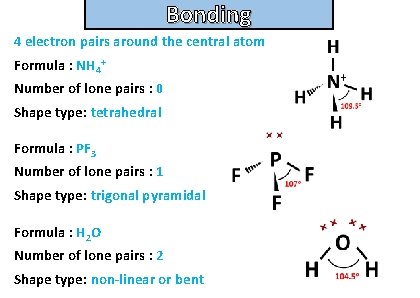
Bonding and structure 4 electron pairs around the central atom Formula : NH 4+ Number of lone pairs : 0 Shape type: tetrahedral Formula : PF 3 Number of lone pairs : 1 Shape type: trigonal pyramidal Formula : H 2 O Number of lone pairs : 2 Shape type: non-linear or bent

Bonding and structure For the following molecules state the number of electron pairs around the central atom, the number of lone pairs and their shape type. Then draw the molecule: • pcl 5 • sf 4 • clf 3
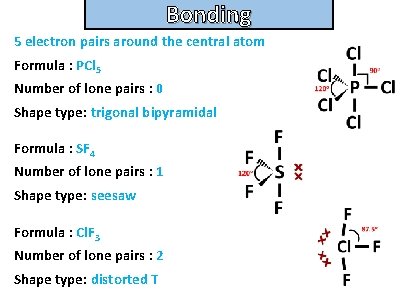
Bonding and structure 5 electron pairs around the central atom Formula : PCl 5 Number of lone pairs : 0 Shape type: trigonal bipyramidal Formula : SF 4 Number of lone pairs : 1 Shape type: seesaw Formula : Cl. F 3 Number of lone pairs : 2 Shape type: distorted T

Bonding and structure For the following molecules state the number of electron pairs around the central atom, the number of lone pairs and their shape type. Then draw the molecule: • sf 6 • if 5 • xef 4
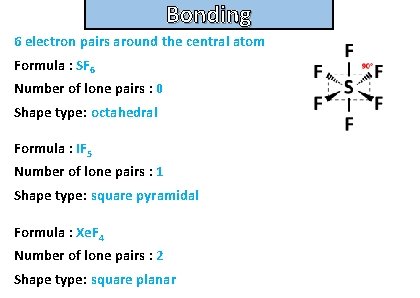
Bonding and structure 6 electron pairs around the central atom Formula : SF 6 Number of lone pairs : 0 Shape type: octahedral Formula : IF 5 Number of lone pairs : 1 Shape type: square pyramidal Formula : Xe. F 4 Number of lone pairs : 2 Shape type: square planar
draw a table to show you can determine the shapes of molecules
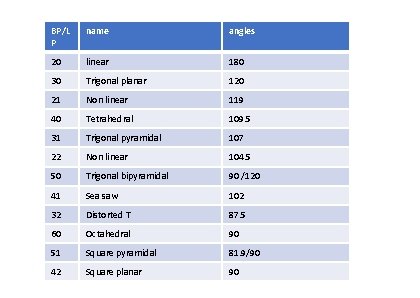
BP/L P name angles 20 linear 180 30 Trigonal planar 120 21 Non linear 119 40 Tetrahedral 109. 5 31 Trigonal pyramidal 107 22 Non linear 104. 5 50 Trigonal bipyramidal 90 /120 41 Sea saw 102 32 Distorted T 87. 5 60 Octahedral 90 51 Square pyramidal 81. 9/90 42 Square planar 90

What is electronegativity ?

Electronegativity is the ability of an atom to attract the • bonding electrons • in a covalent bond

Bonding and structure State and explain the trend in electronegativity of elements seen in the periodic table
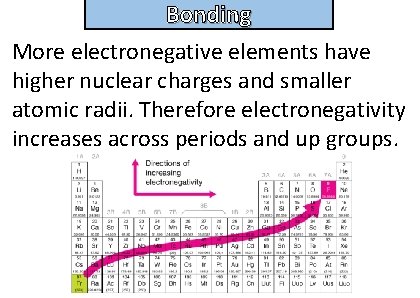
Bonding and structure More electronegative elements have higher nuclear charges and smaller atomic radii. Therefore electronegativity increases across periods and up groups.

Bonding and structure What is a non-polar molecule ?
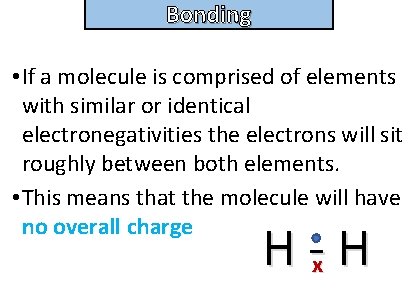
Bonding and structure • If a molecule is comprised of elements with similar or identical electronegativities the electrons will sit roughly between both elements. • This means that the molecule will have no overall charge H -x H

Bonding and structure Give examples of non – polar molecules
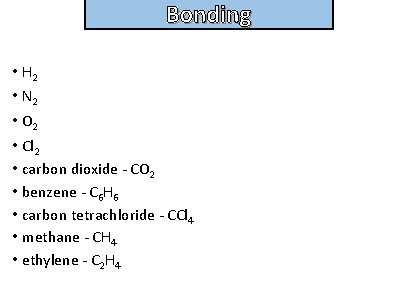
Bonding and structure • H 2 • N 2 • O 2 • Cl 2 • carbon dioxide - CO 2 • benzene - C 6 H 6 • carbon tetrachloride - CCl 4 • methane - CH 4 • ethylene - C 2 H 4

Bonding and structure What is a polar molecule ?
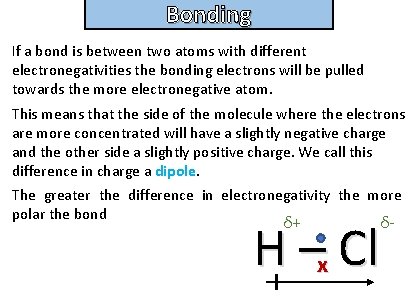
Bonding and structure If a bond is between two atoms with different electronegativities the bonding electrons will be pulled towards the more electronegative atom. This means that the side of the molecule where the electrons are more concentrated will have a slightly negative charge and the other side a slightly positive charge. We call this difference in charge a dipole. The greater the difference in electronegativity the more polar the bond δ+ δ- H –x Cl

Bonding and structure Give examples of polar molecules
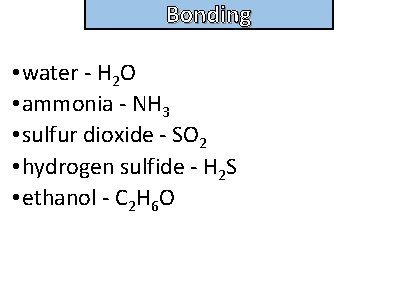
Bonding and structure • water - H 2 O • ammonia - NH 3 • sulfur dioxide - SO 2 • hydrogen sulfide - H 2 S • ethanol - C 2 H 6 O

Bonding and structure How can electronegativity values be used to predict the type of bonding between atoms?

Bonding and structure • A covalent bond has 0% ionic character, and a perfect ionic bond would have 100% ionic character. • The higher the difference in electronegativity between atoms the greater the % ionic character of the bond is • Bonds are polar if the difference in electronegativity value is more than 0. 4 • % ionic character is determined by looking at the following table from your data booklet: Electronegativity difference 0. 1 0. 3 0. 5 0. 7 1. 0 1. 3 1. 5 1. 7 2. 0 2. 5 3. 0 % ionic character 0. 5 2 6 12 22 34 43 51 63 79 89

Bonding and structure Calculate the percent ionic character of li-Cl given that the Pauling electronegativity values of li = 2. 0 and Cl = 3. 0
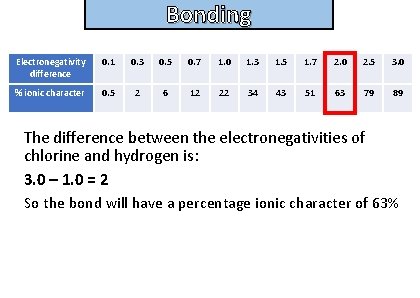
Bonding and structure Electronegativity difference 0. 1 0. 3 0. 5 0. 7 1. 0 1. 3 1. 5 1. 7 2. 0 2. 5 3. 0 % ionic character 0. 5 2 6 12 22 34 43 51 63 79 89 The difference between the electronegativities of chlorine and hydrogen is: 3. 0 – 1. 0 = 2 So the bond will have a percentage ionic character of 63%

Bonding and structure State the electronegativity difference ranges for a molecule to be considered: • Non-polar covalent • Polar covalent • ionic

Bonding and structure • Electronegativity difference equal to 0 then its nonpolar • Electronegativity difference is more than 0 but less than 1. 7 are polar covalent • Electronegativity difference greater than 1. 7 are ionic

Explain how we can determine if a molecule is polar

δ+ δ- In a simple molecule such as HCl, the polar bond gives the whole molecule a permanent dipole. It’s a polar molecule H---Cl x If polar bonds point in opposite directions they cancel each other out. The molecule is non-polar δ- If the polar bonds all point in roughly the same direction ( bonds arranged symmetrically) then the molecule will be polar δ+ δ- O=C=C δ+ δ- δ- δ-
Bonding and structure What are intermolecular forces ?
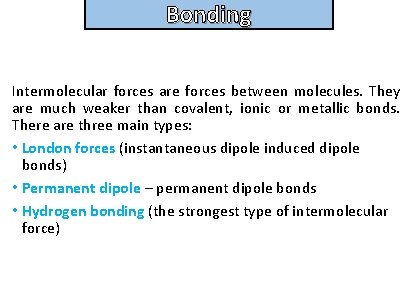
Bonding and structure Intermolecular forces are forces between molecules. They are much weaker than covalent, ionic or metallic bonds. There are three main types: • London forces (instantaneous dipole induced dipole bonds) • Permanent dipole – permanent dipole bonds • Hydrogen bonding (the strongest type of intermolecular force)

Bonding and structure Describe the nature of London forces

Bonding and structure • London forces cause all atoms and molecules to be attracted to each other. • At any one time the electrons in an atom are likely to be more on one side of the atom than the other creating a temporary dipole. • These dipoles can induce other dipoles in neighboring atoms • Because electrons are always moving these dipoles are created and destroyed all the time but the overall effect is for the atoms to be attracted to each other

Bonding What is the role of London forces in covalent molecules
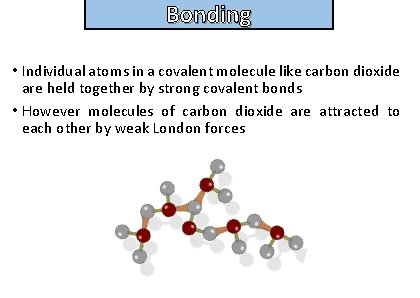
Bonding and structure • Individual atoms in a covalent molecule like carbon dioxide are held together by strong covalent bonds • However molecules of carbon dioxide are attracted to each other by weak London forces

Bonding What factors effect the strength of London forces and therefore melting and boiling points of substances ?
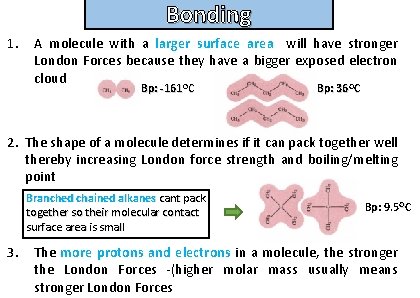
Bonding and structure 1. A molecule with a larger surface area will have stronger London Forces because they have a bigger exposed electron cloud O O Bp: -161 C Bp: 36 C 2. The shape of a molecule determines if it can pack together well thereby increasing London force strength and boiling/melting point Branched chained alkanes cant pack together so their molecular contact surface area is small 3. Bp: 9. 5 OC The more protons and electrons in a molecule, the stronger the London Forces -(higher molar mass usually means stronger London Forces

Bonding Describe the nature of permanent dipole – permanent dipole bonds
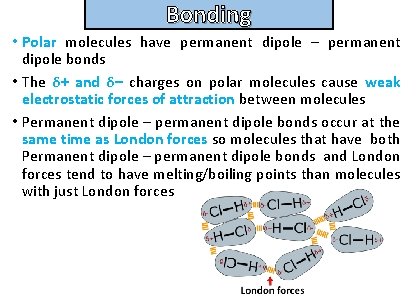
Bonding and structure • Polar molecules have permanent dipole – permanent dipole bonds • The δ+ and δ– charges on polar molecules cause weak electrostatic forces of attraction between molecules • Permanent dipole – permanent dipole bonds occur at the same time as London forces so molecules that have both Permanent dipole – permanent dipole bonds and London forces tend to have melting/boiling points than molecules with just London forces

Bonding Describe the nature of hydrogen bonding
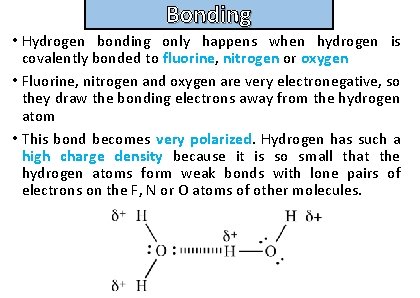
Bonding and structure • Hydrogen bonding only happens when hydrogen is covalently bonded to fluorine, nitrogen or oxygen • Fluorine, nitrogen and oxygen are very electronegative, so they draw the bonding electrons away from the hydrogen atom • This bond becomes very polarized. Hydrogen has such a high charge density because it is so small that the hydrogen atoms form weak bonds with lone pairs of electrons on the F, N or O atoms of other molecules.

Bonding Name 3 molecules that undergo hydrogen bonding

Bonding and structure 1. Water 2. Ammonia 3. Hydrogen fluoride

Bonding Organic molecules that form hydrogen bonds often contain what functional groups ?
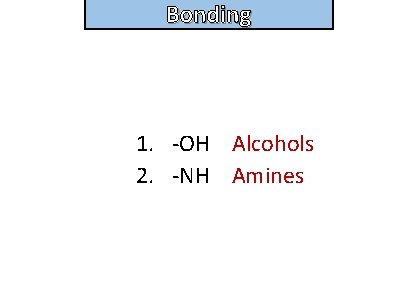
Bonding and structure 1. -OH Alcohols 2. -NH Amines
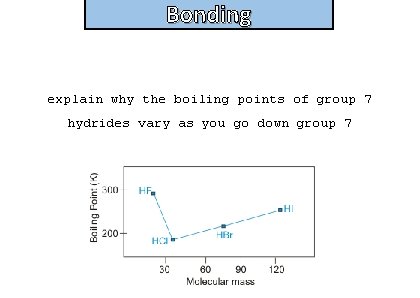
Bonding explain why the boiling points of group 7 hydrides vary as you go down group 7

Bonding and structure • Substances that form H bonds have high melting and boiling points because a lot of energy is needed to overcome the intermolecular forces • From HCl to HBr to HI, the halogen atom is getting bigger (bigger molar mass)with more electrons. This increases the size of the London forces • The permanent polarity of these molecules is falling, because the halogens get less electronegative from Cl to Br to I, and so there is less electronegativity difference between the halogen and the hydrogen. The permanent dipole-dipole attractions therefore fall – but not enough to counteract the effect of the increasing London forces. So the boiling point increases from HCl to HBr to HI • HF has a higher boiling point than HCl, HBr or HI because the highly electronegative F results in hydrogen bonds being

Bonding Write down the different intermolecular forces in order of strength from lowest to highest
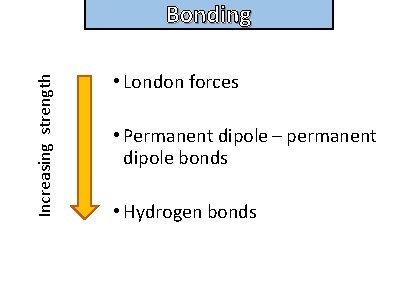
Increasing strength Bonding and structure • London forces • Permanent dipole – permanent dipole bonds • Hydrogen bonds

Bonding explain why the boiling points of group 6 hydrides vary as you go down group 7

Bonding and structure • H 2 O has a relatively high boiling point when compared to other group 6 hydrides due to the presence of hydrogen bonds • The boiling points from H 2 S to H 2 Se to H 2 Te increase due to increasing London forces which override the decrease in strength of the permanent dipole – permanent dipole bonds

Describe how the presence of hydrogen bonds explain why ice is less dense than liquid water
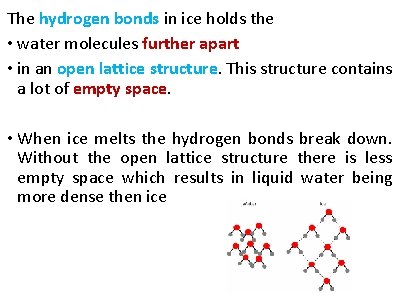
The hydrogen bonds in ice holds the • water molecules further apart • in an open lattice structure. This structure contains a lot of empty space. • When ice melts the hydrogen bonds break down. Without the open lattice structure there is less empty space which results in liquid water being more dense then ice

Bonding explain why alcohols are less volatile than similar alkanes ?

Bonding and structure • Alcohols contain a polar hydroxyl group (-OH) which can form hydrogen bonds • Hydrogen bonding gives alcohols low volatilities compared to other alkanes with similar numbers of electrons

Bonding What 3 things must occur in order for one substance to dissolve in another ?

Bonding and structure • Bonds in the substance have to break • Bonds in the solvent have to break • New bonds have to form between the substance and the solvent

Bonding What condition must be met in order for a substance to dissolve ?

Bonding and structure • A substance will only dissolve if the strength of the new bonds formed is about the same as, or greater than the strength of the bonds that are broken

Bonding Describe the two main types of solvent and give examples

Bonding and structure 1. Polar solvents • Made of polar molecules • Water bonds together via hydrogen bonds • Propanone (acetone) only forms London forces and permanent dipole – permanent dipole bonds 2. Non – polar solvents • Made of non - polar molecules • Hexane molecules bond to each other by London forces

Bonding Describe the process of hydration
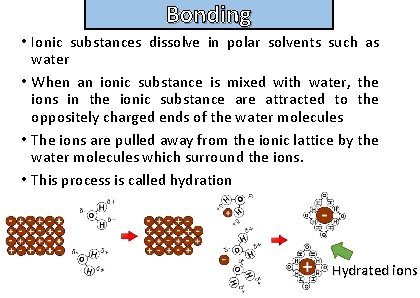
Bonding and structure • Ionic substances dissolve in polar solvents such as water • When an ionic substance is mixed with water, the ions in the ionic substance are attracted to the oppositely charged ends of the water molecules • The ions are pulled away from the ionic lattice by the water molecules which surround the ions. • This process is called hydration Hydrated ions

Bonding Why don’t some ionic substances dissolve in polar solvents such as water ?

Bonding and structure • Some ionic substances don’t dissolve because the bonding between their ions is too strong. • For example aluminium oxide (Al 2 O 3) is insoluble in water because the bonds between the ions are stronger than the bonds they would form with the water molecules

Bonding Explain why alcohols dissolve in polar solvents such as water

Bonding and structure • Although alcohols are covalent they still dissolve in water • The polar O-H bond in an alcohol is attracted to the polar O-H bonds in water. • Hydrogen bonds form between the lone pairs on the δ- oxygen atoms and the δ+ hydrogen atoms

Bonding Why are alcohols with longer carbon lengths less soluble in water ? chain

Bonding and structure The carbon chain part of the alcohol is not attracted to water so the more carbon atoms there are the less soluble the alcohol will be

Bonding Explain why not all molecules with polar bonds dissolve in water

Bonding and structure 1. Halogenoalkanes contain polar bonds but their dipoles are not strong enough to form hydrogen bonds with water 2. The hydrogen bonding between water molecules is stronger than the bonds that would be formed with halogenoalkanes, so halogenoalkanes don’t dissolve

Bonding In what type of solvent do halogenoalkanes dissolve

Bonding and structure Halogenoalkanes can form permanent dipole-dipole bonds. They will therefore dissolve in polar solvents that also form permanent dipole-dipole bonds

Bonding What determines the properties of a solid ?

Bonding and structure Melting and boiling point of a substance are determined by the strength of attraction due to its intermolecular forces A substance only conducts electricity if it contains charged particles that are free to move Substances that are able to form hydrogen bonds or contain charged ions will dissolve in water, whereas non-polar or uncharged substances do not

Bonding State the properties of ionic compounds
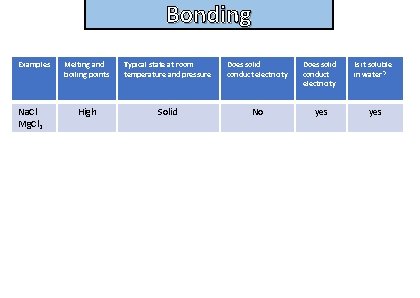
Bonding and structure Examples Na. Cl Mg. Cl 2 Melting and boiling points Typical state at room temperature and pressure Does solid conduct electricity Is it soluble in water? High Solid No yes
Bonding State the properties of simple covalent (molecular) molecules
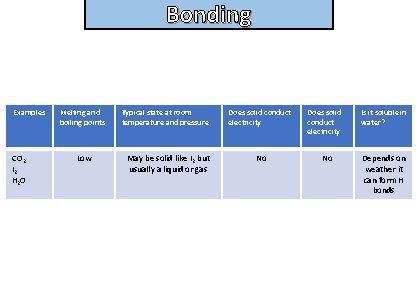
Bonding and structure Examples CO 2 I 2 H 2 O Melting and boiling points Low Typical state at room temperature and pressure May be solid like I 2 but usually a liquid or gas Does solid conduct electricity No Is it soluble in water? Depends on weather it can form H bonds
Bonding State the properties of simple giant covalent compounds
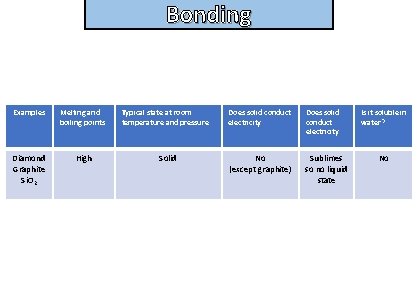
Bonding and structure Examples Melting and boiling points Diamond Graphite Si. O 2 High Typical state at room temperature and pressure Solid Does solid conduct electricity No (except graphite) Sublimes so no liquid state Is it soluble in water? No

Bonding State the properties of simple metallic compounds
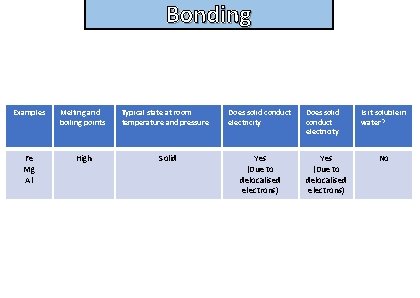
Bonding and structure Examples Melting and boiling points Fe Mg Al High Typical state at room temperature and pressure Solid Does solid conduct electricity Yes (Due to delocalised electrons) Is it soluble in water? No

Bonding Iodine, I 2 and graphite are both solid at r. t. p. At 500 K, iodine exists as a gas , while graphite remains solid. Explain this difference in the properties of iodine their structures and graphite in terms of

Bonding and structure • Iodine is a simple molecular substance • To melt or boil iodine you only need to overcome the weak intermolecular forces holding the molecules together, which doesn’t need much energy • Graphite is a giant covalent substance • Graphite will remain solid unless you can overcome the strong covalent bonds between atoms, which needs a lot of energy

Bonding A substance, X, has a melting point of 650 o. C and a boiling point of 1107 o. C. It conducts electricity in both the solid and liquid states but is insoluble in water. Which of the following could be substance X? 1. Carbon dioxide 2. magnesium 3. caesium chloride 4. silicon dioxide

Bonding and structure B
Bonding State three substances that giant lattices are present in

Bonding and structure 1. ionic solids (giant ionic lattices) 2. covalently bonded solids, such as diamond, graphite and silicon(IV) oxide (giant covalent lattices) 3. solid metals (giant metallic lattices)

Bonding Describe the lattice structure of diamond , and silicon dioxide
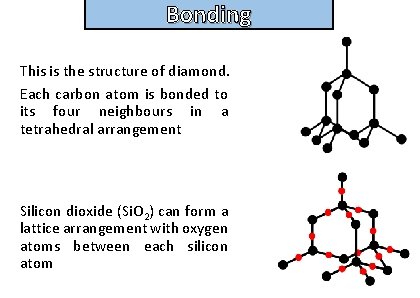
Bonding and structure This is the structure of diamond. Each carbon atom is bonded to its four neighbours in a tetrahedral arrangement Silicon dioxide (Si. O 2) can form a lattice arrangement with oxygen atoms between each silicon atom

How do the properties of giant structures provide evidence for covalent bonding ?

1. Have very high melting points because: • Many covalent bonds which are strong • Breaking these bonds requires a lot of energy 1. Are usually extremely hard due to strong bonds through out the lattice structure 2. Are good thermal conductors – vibrations travel easily through the stiff lattices 3. They are insoluble - the covalent bonds mean atoms are more attracted to their neighbours in the lattice than to solvent molecules. The fact that they are insoluble in polar solvents show that they don’t contain ions 4. Don’t conduct electricity • electrons are fixed in covalent bonds • so there are no charged ions or free electrons
Bonding Explain how graphite can conduct electricity
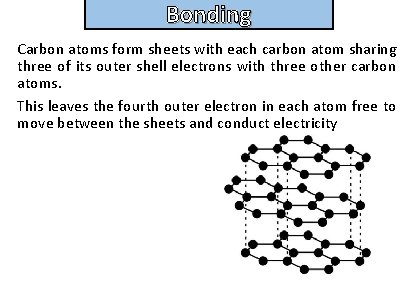
Bonding and structure Carbon atoms form sheets with each carbon atom sharing three of its outer shell electrons with three other carbon atoms. This leaves the fourth outer electron in each atom free to move between the sheets and conduct electricity

Bonding Describe the structure and state the properties of graphene

Bonding and structure Graphene is a sheet of carbon atoms joined together in hexagons The sheet is just one atom thick making it a 2 – dimensional compound Graphene's structure means it can 1. Conduct electricity 2. Very strong 3. Transparent 4. Very light
Explain what is meant by metallic structure and bonding. Draw a diagram to illustrate your answer

• Structure: • Lattice of positive ions in a sea of delocalised electrons • bonding results from the attraction between positive metal ions and a sea of delocalised electrons between them

Bonding How does the metallic bonding model explain the high melting point of metals ?

High melting point due to strong metallic bonding The more delocalised electrons the stronger this bond so Mg 2+ has a higher melting point than Na+ because it has: 1. A larger charge density than Na+ 2. Mg 2+ is smaller than Na+ 3. 2 free electrons per atom to contribute to the sea of electrons instead of just 1 in Na+ 4. Mg 2+ has a greater attraction for the sea of delocalised electrons
Bonding How does the metallic bonding model explain why metals are both malleable and ductile ?

Bonding and structure As there are no bonds holding specific ions together and the layers of positive metal ions are separated by layers of electrons, metals are malleable (can be shaped) and are ductile (can be drawn into a wire). The layers of metal ions can slide over each other without disrupting the attraction between the positive ions and electrons
Bonding How does the metallic bonding model explains why metals are good thermal conductors ?

Bonding and structure The delocalised electrons can pass kinetic energy to each other making metals good thermal conductors
Bonding How does the metallic bonding model explains why metals are good electrical conductors ?

Bonding and structure Metals are good electrical conductors because: • the delocalised electrons • are free to move and can carry a current. Any impurities can dramatically reduce electrical conductivity by reducing the number of electrons that are free to move and carry charge – the electrons transfer to the impurities and form anions
Bonding Why are metals insoluble ?

Bonding and structure Metals are insoluble, except in liquid metals because of the strength of the metallic bonds
- Slides: 164