Bonding An Introduction What Is A Chemical Bond

Bonding An Introduction
What Is A Chemical Bond? A force that holds groups of two or more atoms together
Principle of All Bondings • Elements try to attain noble gas electron configuration by losing, gaining, or sharing electrons. • Noble Gases (in Group 18) – All have 8 valence electrons in their outermost energy level (except He). This is called an octet. – These elements are not likely to lose, gain, or share electrons because they have a very stable electron configuration. • Other elements form bonds to try to get a similar electron configuration (a stable octet of valence electrons).
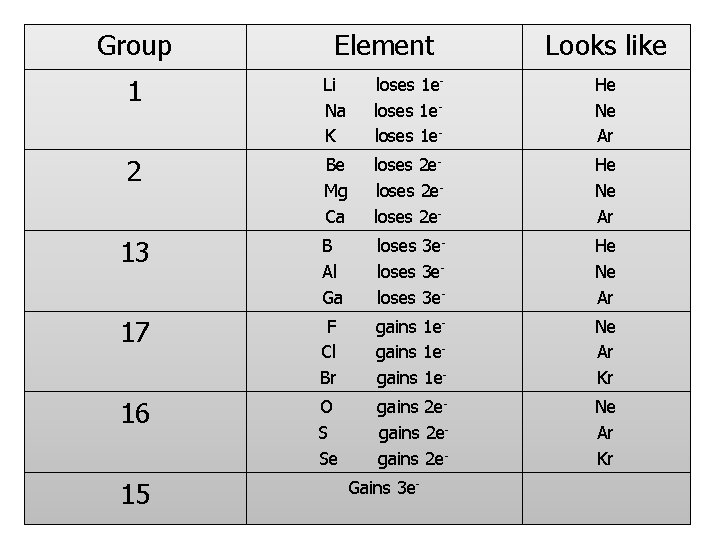
Group Element Looks like 1 Li Na K loses 1 eloses 1 e- He Ne Ar 2 Be Mg Ca loses 2 eloses 2 e- He Ne Ar 13 B Al Ga loses 3 eloses 3 e- He Ne Ar 17 F Cl Br gains 1 egains 1 e- Ne Ar Kr 16 O S Se gains 2 egains 2 e- Ne Ar Kr 15 Gains 3 e-
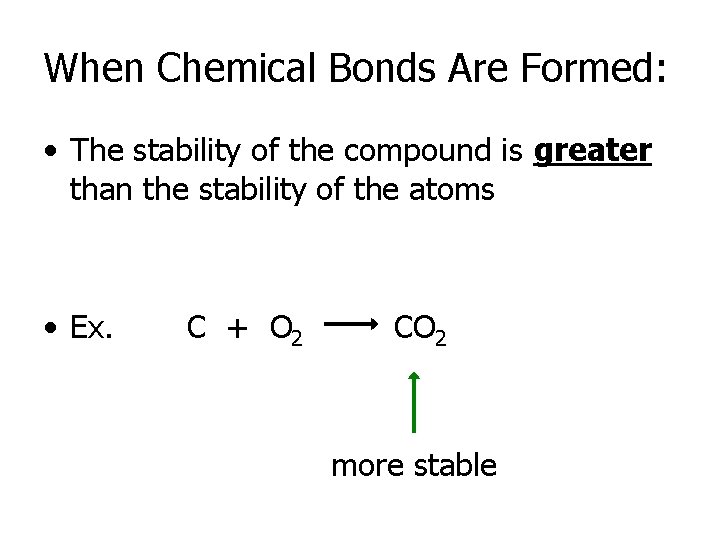
When Chemical Bonds Are Formed: • The stability of the compound is greater than the stability of the atoms • Ex. C + O 2 CO 2 more stable
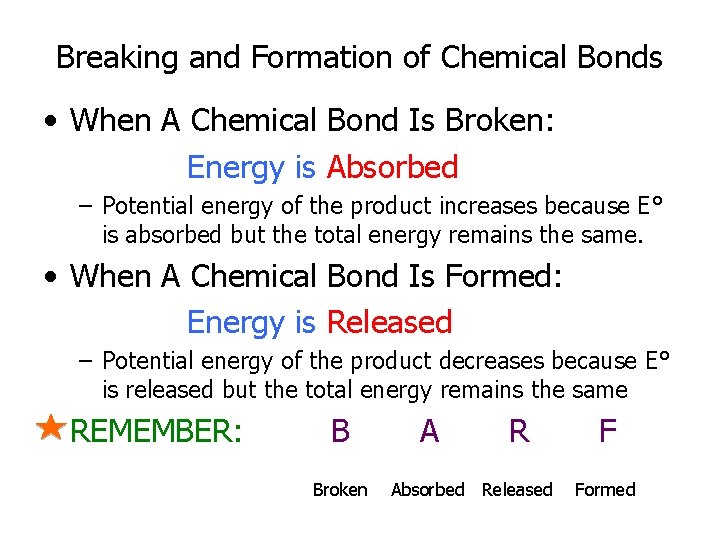
Breaking and Formation of Chemical Bonds • When A Chemical Bond Is Broken: Energy is Absorbed – Potential energy of the product increases because E° is absorbed but the total energy remains the same. • When A Chemical Bond Is Formed: Energy is Released – Potential energy of the product decreases because E° is released but the total energy remains the same REMEMBER: B Broken A R Absorbed Released F Formed
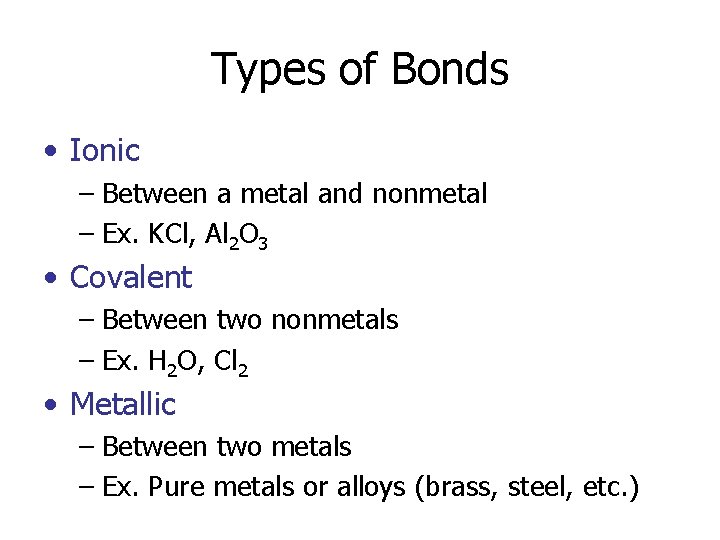
Types of Bonds • Ionic – Between a metal and nonmetal – Ex. KCl, Al 2 O 3 • Covalent – Between two nonmetals – Ex. H 2 O, Cl 2 • Metallic – Between two metals – Ex. Pure metals or alloys (brass, steel, etc. )
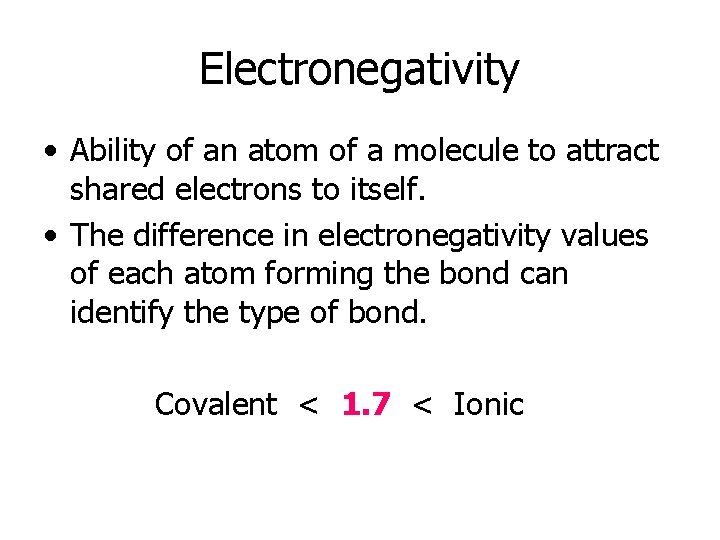
Electronegativity • Ability of an atom of a molecule to attract shared electrons to itself. • The difference in electronegativity values of each atom forming the bond can identify the type of bond. Covalent < 1. 7 < Ionic
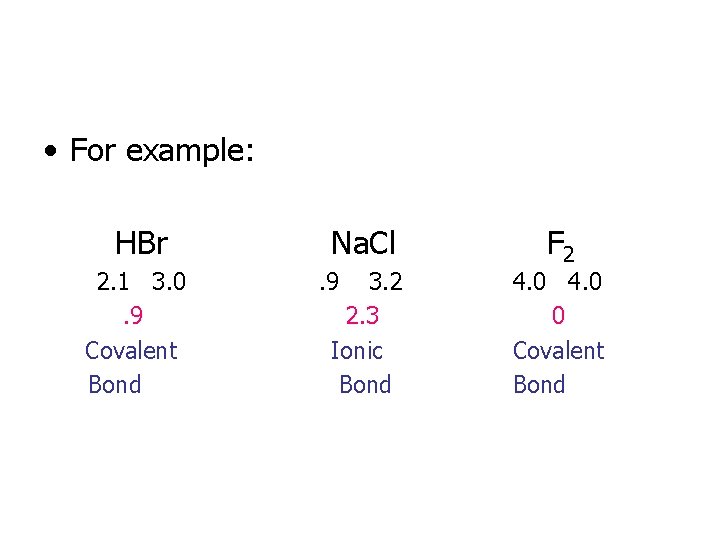
• For example: HBr 2. 1 3. 0. 9 Covalent Bond Na. Cl. 9 3. 2 2. 3 Ionic Bond F 2 4. 0 0 Covalent Bond
Bonding Ionic Bonds & Characteristics of Ionic Compounds
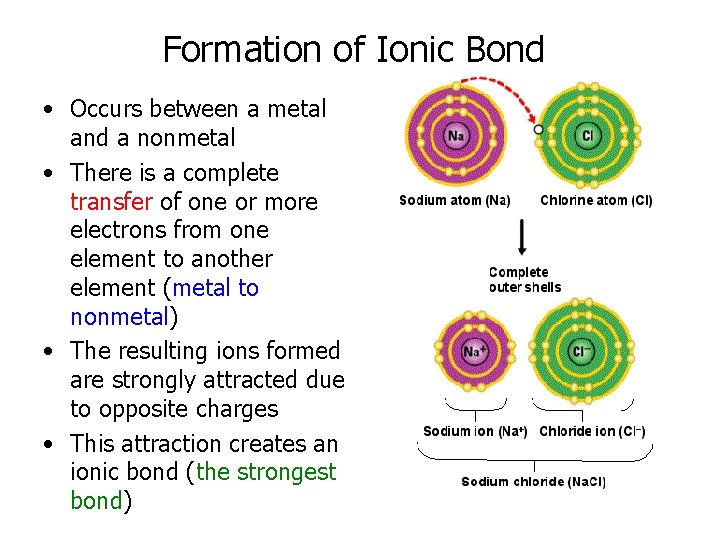
Formation of Ionic Bond • Occurs between a metal and a nonmetal • There is a complete transfer of one or more electrons from one element to another element (metal to nonmetal) • The resulting ions formed are strongly attracted due to opposite charges • This attraction creates an ionic bond (the strongest bond)
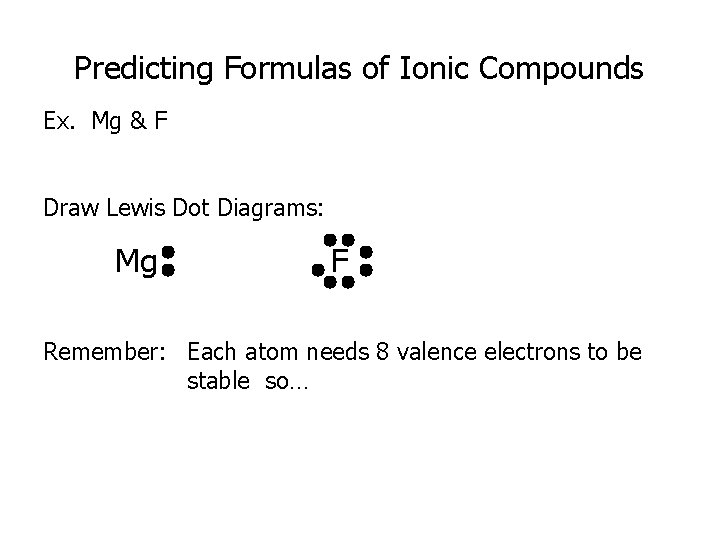
Predicting Formulas of Ionic Compounds Ex. Mg & F Draw Lewis Dot Diagrams: Mg F Remember: Each atom needs 8 valence electrons to be stable so…
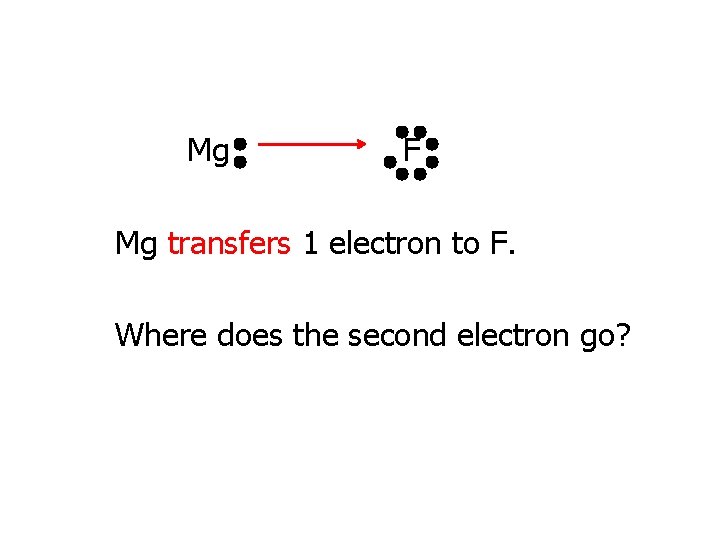
Mg F Mg transfers 1 electron to F. Where does the second electron go?
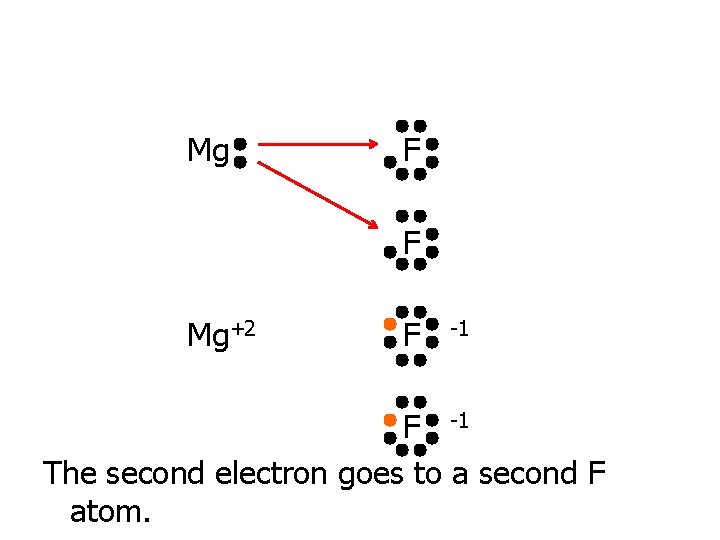
Mg F F Mg+2 F -1 The second electron goes to a second F atom.
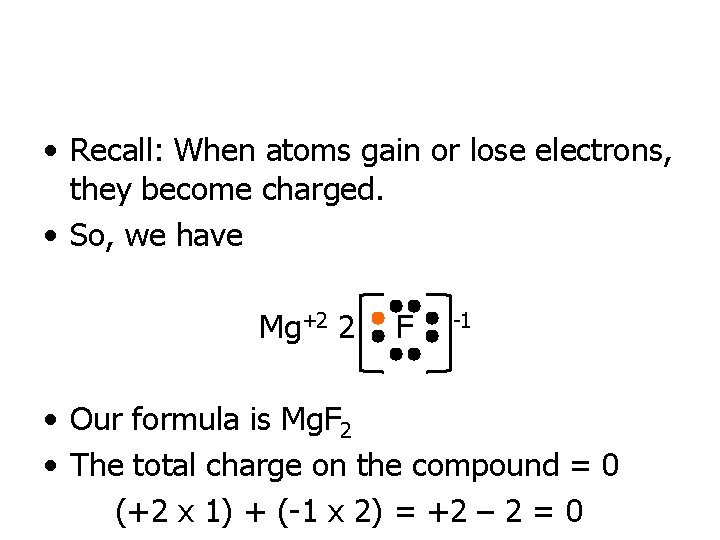
• Recall: When atoms gain or lose electrons, they become charged. • So, we have Mg+2 2 F -1 • Our formula is Mg. F 2 • The total charge on the compound = 0 (+2 x 1) + (-1 x 2) = +2 – 2 = 0

Try These! 1. K and O 1. Mg and N 3. Ca and Cl
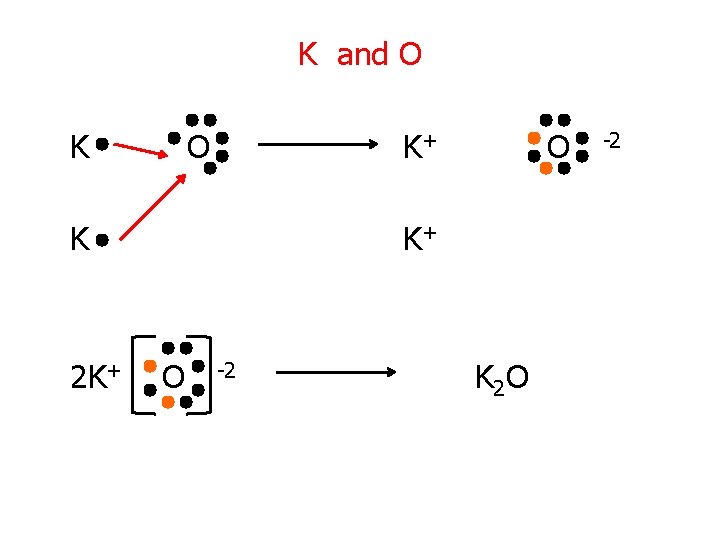
K and O K+ K 2 K+ O -2 K 2 O -2
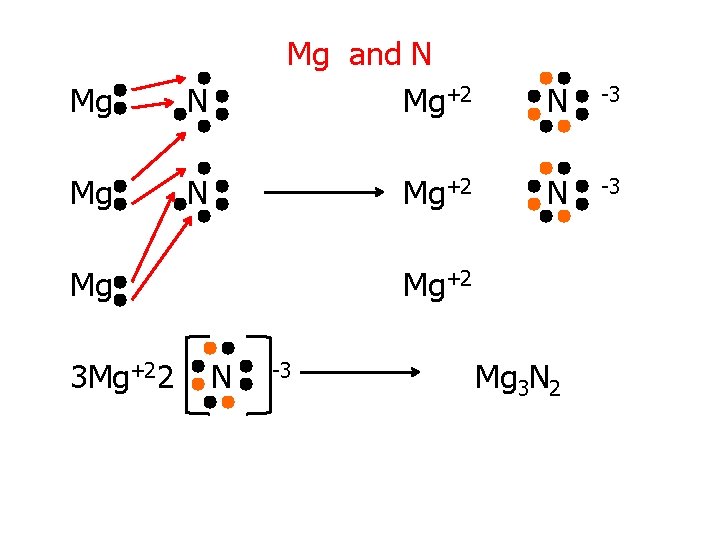
Mg N Mg and N Mg+2 Mg 3 Mg+22 N -3 Mg+2 N -3 Mg 3 N 2
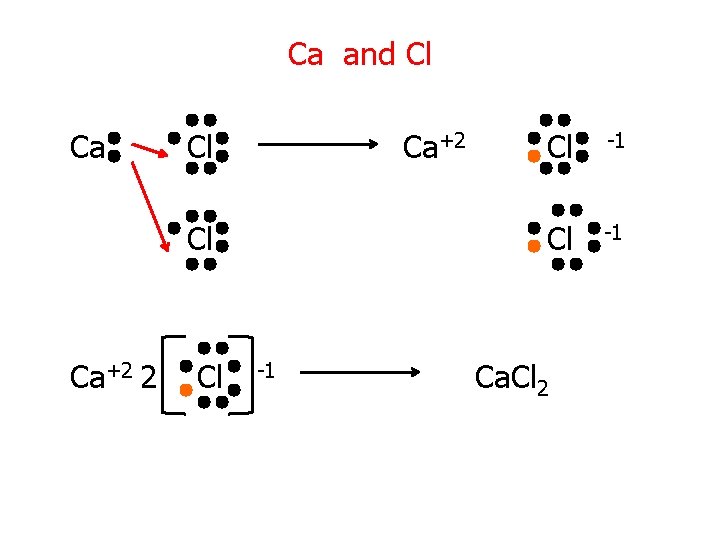
Ca and Cl Ca+2 2 Cl -1 Ca. Cl 2
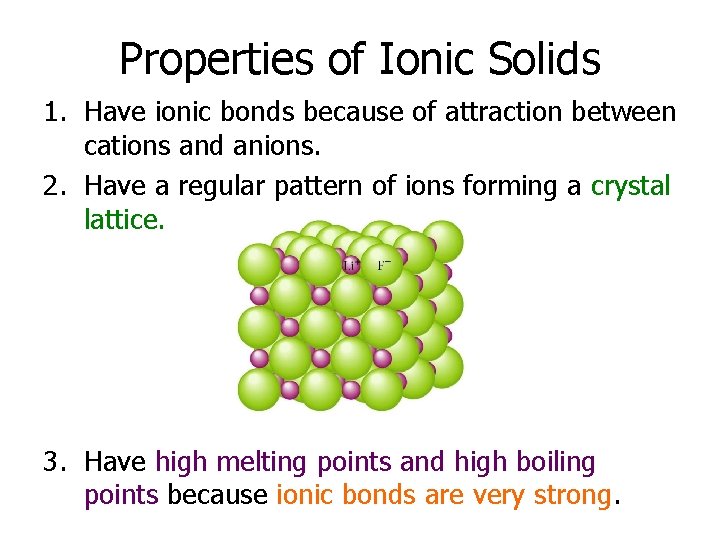
Properties of Ionic Solids 1. Have ionic bonds because of attraction between cations and anions. 2. Have a regular pattern of ions forming a crystal lattice. 3. Have high melting points and high boiling points because ionic bonds are very strong.
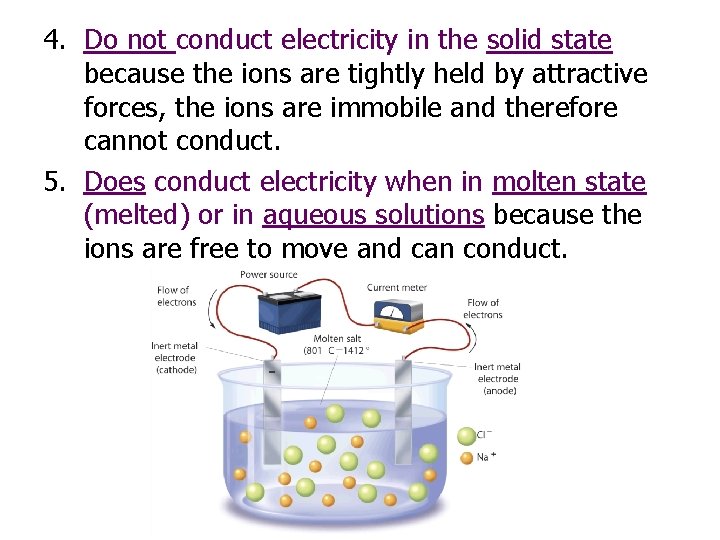
4. Do not conduct electricity in the solid state because the ions are tightly held by attractive forces, the ions are immobile and therefore cannot conduct. 5. Does conduct electricity when in molten state (melted) or in aqueous solutions because the ions are free to move and can conduct.
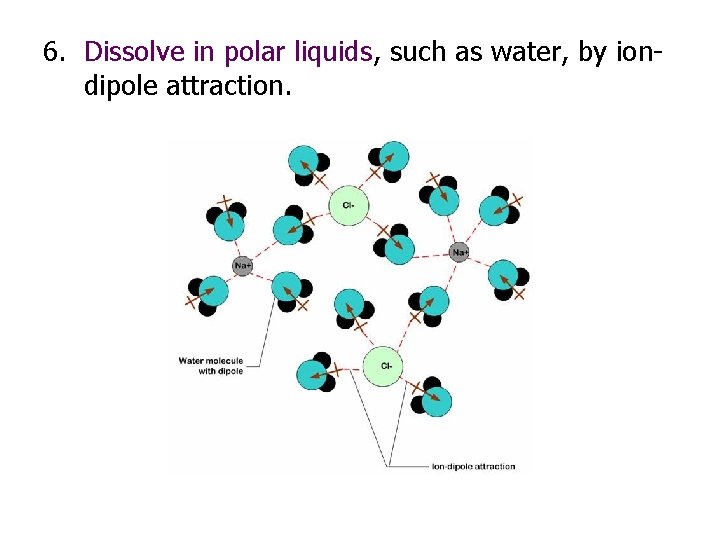
6. Dissolve in polar liquids, such as water, by iondipole attraction.
Bonding Covalent Bonds & Characteristics of Covalent Compounds
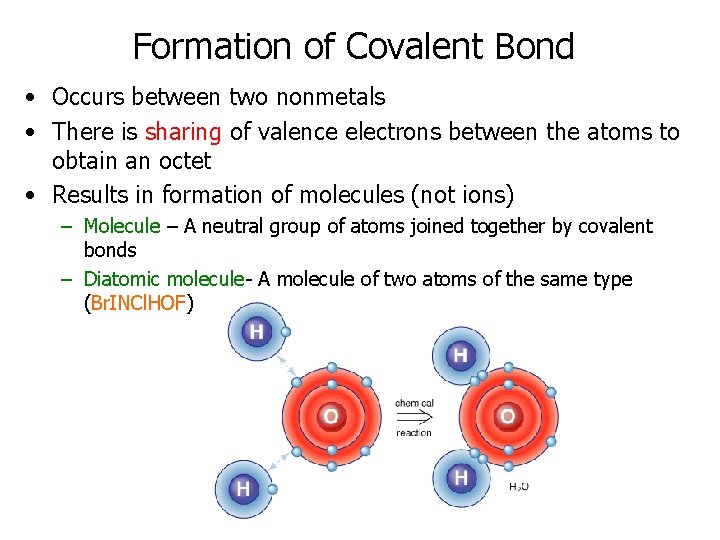
Formation of Covalent Bond • Occurs between two nonmetals • There is sharing of valence electrons between the atoms to obtain an octet • Results in formation of molecules (not ions) – Molecule – A neutral group of atoms joined together by covalent bonds – Diatomic molecule- A molecule of two atoms of the same type (Br. INCl. HOF)
Steps for Lewis Dot Structures of Covalent Compounds 1. Determine the total number of valence electrons in molecule 2. Place the least electronegative atom in the center 3. Draw single bonds between the central and terminal atoms (each single bond = 2 e-) 4. Complete the octets of all terminal atoms with remaining valence electrons 5. If the central atom does not have a complete octet, you must move a pair of electrons from a terminal atom to the central atom to make a multiple bond
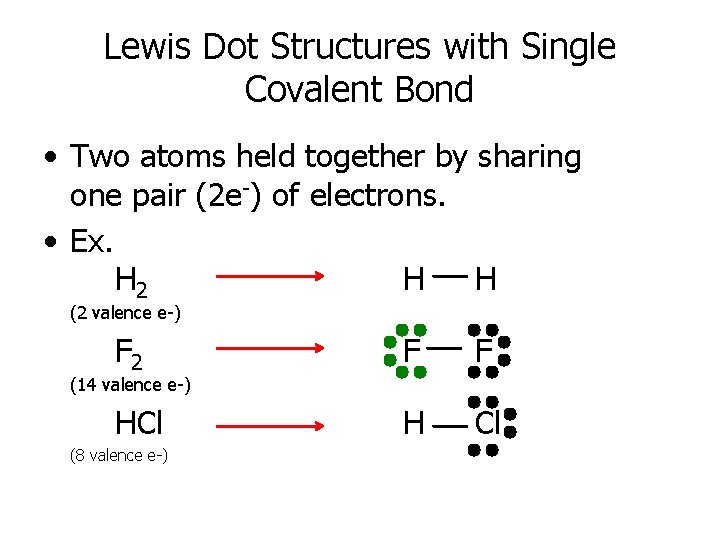
Lewis Dot Structures with Single Covalent Bond • Two atoms held together by sharing one pair (2 e-) of electrons. • Ex. H 2 H H (2 valence e-) F 2 F F HCl H Cl (14 valence e-) (8 valence e-)
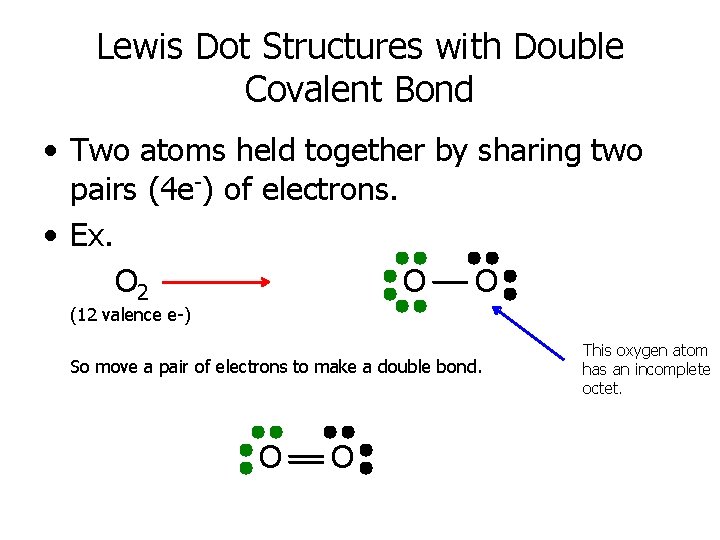
Lewis Dot Structures with Double Covalent Bond • Two atoms held together by sharing two pairs (4 e-) of electrons. • Ex. O 2 O O (12 valence e-) So move a pair of electrons to make a double bond. O O This oxygen atom has an incomplete octet.
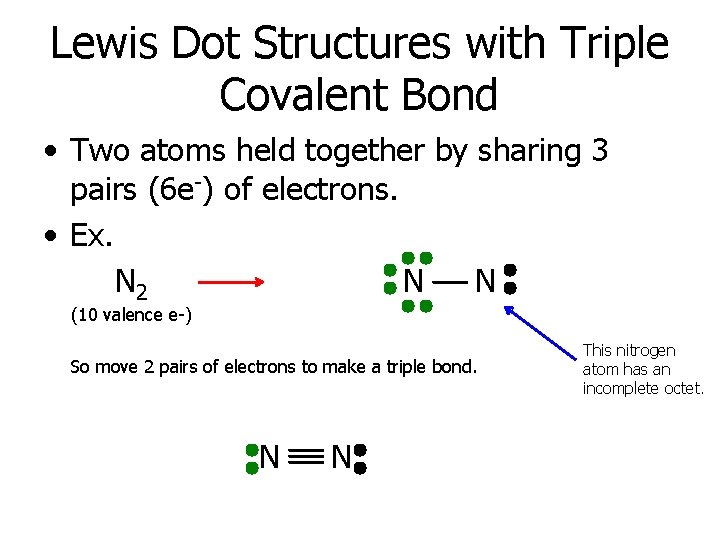
Lewis Dot Structures with Triple Covalent Bond • Two atoms held together by sharing 3 pairs (6 e-) of electrons. • Ex. N 2 N N (10 valence e-) So move 2 pairs of electrons to make a triple bond. N N This nitrogen atom has an incomplete octet.

Try These! • H 2 O • NH 3 • CO 2
Polar vs. Nonpolar Covalent Bonds The bonding pairs of electrons in covalent bonds are pulled by the nuclei.
3 Types of Covalent Bonds 1. Nonpolar Covalent Bonds – Equal sharing or equal pull/attraction for electrons – The similar pull on the electrons is due to the same electronegativity values – Shared electrons are in the middle of the 2 nuclei – Molecule formed is nonpolar – Difference in electronegativity is zero
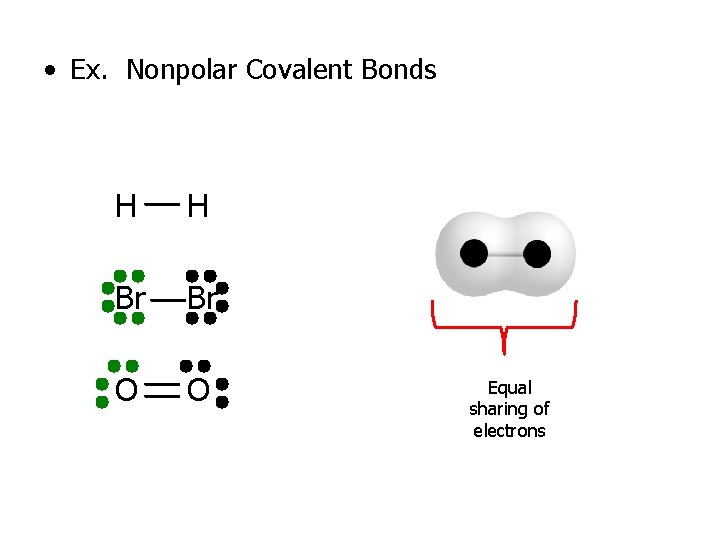
• Ex. Nonpolar Covalent Bonds H H Br Br O O Equal sharing of electrons
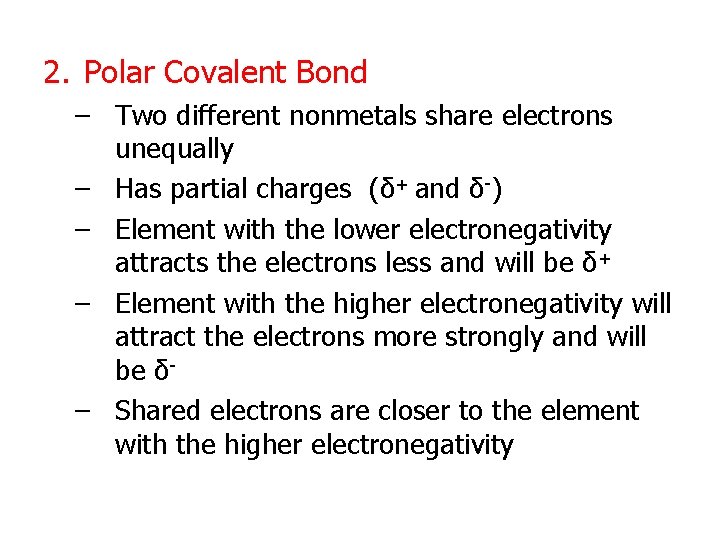
2. Polar Covalent Bond – Two different nonmetals share electrons unequally – Has partial charges (δ+ and δ-) – Element with the lower electronegativity attracts the electrons less and will be δ+ – Element with the higher electronegativity will attract the electrons more strongly and will be δ– Shared electrons are closer to the element with the higher electronegativity
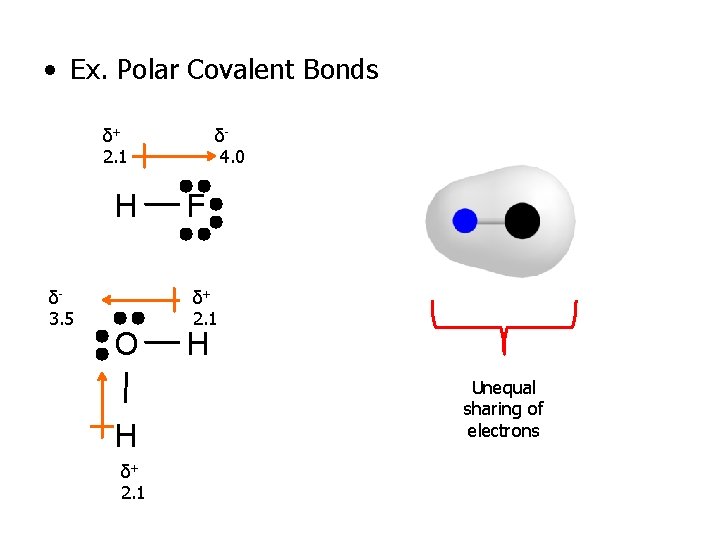
• Ex. Polar Covalent Bonds δ+ 2. 1 H δ 3. 5 O H δ+ 2. 1 δ 4. 0 F δ+ 2. 1 H Unequal sharing of electrons
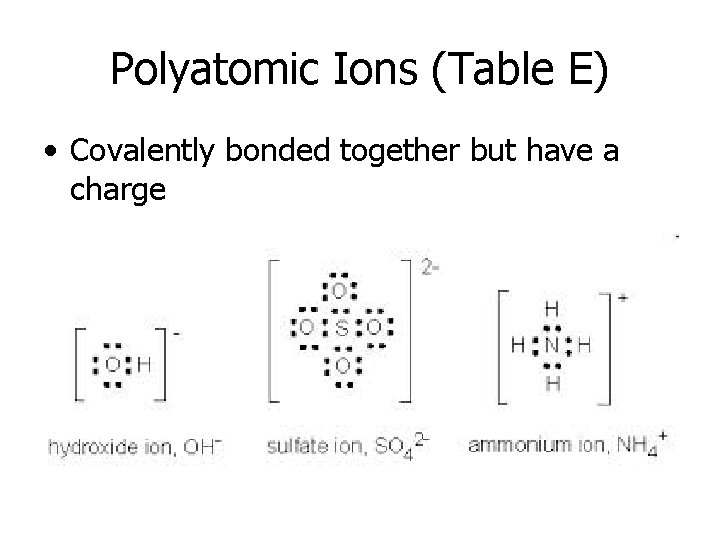
Polyatomic Ions (Table E) • Covalently bonded together but have a charge
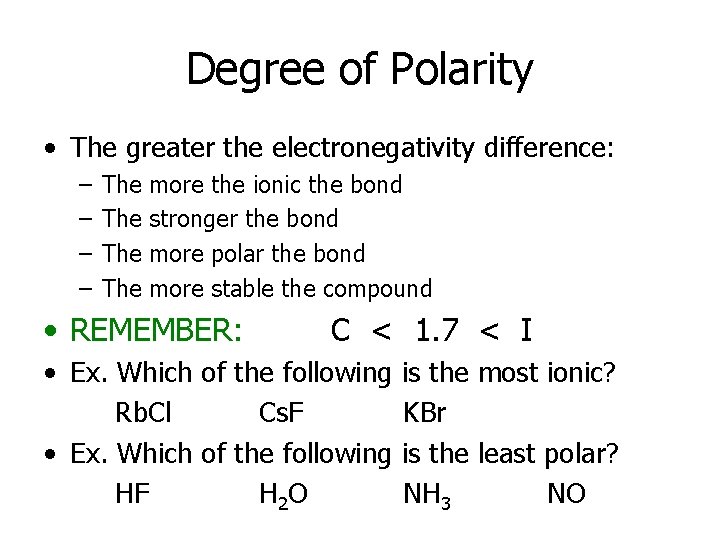
Degree of Polarity • The greater the electronegativity difference: – – The The more the ionic the bond stronger the bond more polar the bond more stable the compound • REMEMBER: C < 1. 7 < I • Ex. Which of the following Rb. Cl Cs. F • Ex. Which of the following HF H 2 O is the most ionic? KBr is the least polar? NH 3 NO
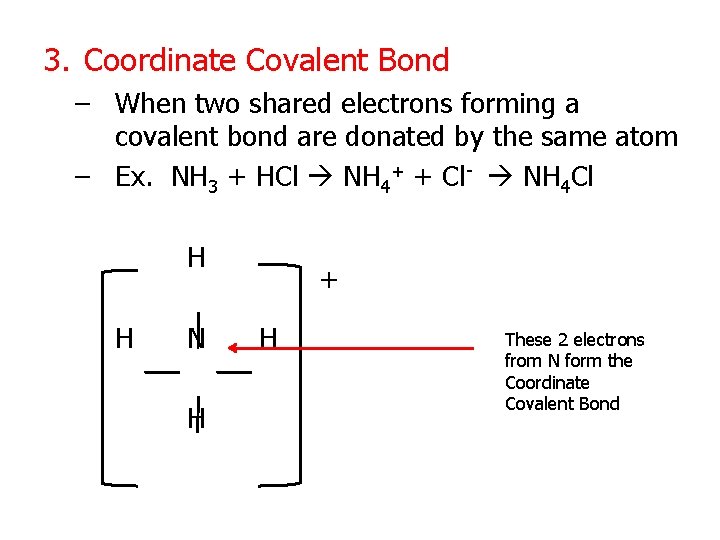
3. Coordinate Covalent Bond – When two shared electrons forming a covalent bond are donated by the same atom – Ex. NH 3 + HCl NH 4+ + Cl- NH 4 Cl H H N H + H These 2 electrons from N form the Coordinate Covalent Bond
Types & Properties of Covalent Solids 1. Molecular Solids – – Soft solids that never conduct electricity Held together by covalent bonds in the molecule (and Vander Waal’s Forces between the molecules) Have low melting and boiling points Ex. Solid CO 2 (dry ice) Solid I 2 (iodine) Sucrose
2. Network Solids – – – Solids in which all of the atoms are covalently bonded to each other (makes them very strong) Hard solids that never conduct electricity Have high melting (do not melt until temp. reaches 1000°C or higher) and boiling points Do not dissolve easily in any liquid Ex. Diamond (allotrope of carbon) » Does not melt; Vaporizes to a gas at 3500°C or above) Si. O 2 (silicon dioxide) Si. C (silicon carbide) » » Sand Has a melting point of about 2700°C)
Bonding Molecular Geometry
Molecular Geometry • Explained by VSPER – Valence Shell Electron-Pair Repulsion model – Based on the repulsive behavior of electron pairs • Bonding Pairs – Those electrons shared by the central atom and any atom to which it is bonded. • Non-Bonding Pairs (lone pairs) – Those pairs of electrons on an individual atom that are not shared with another atom – To determine the geometry (shape) of a molecule: • write the Lewis structure • determine the number of bonding groups of electrons and the number of non-bonding pairs of electrons on the central atom • use the associated name for that shape
Molecular Geometry • The geometry of a molecule is determined by arranging the electron pairs in a way that minimizes repulsions • To do this, electron pairs are placed as far apart as possible
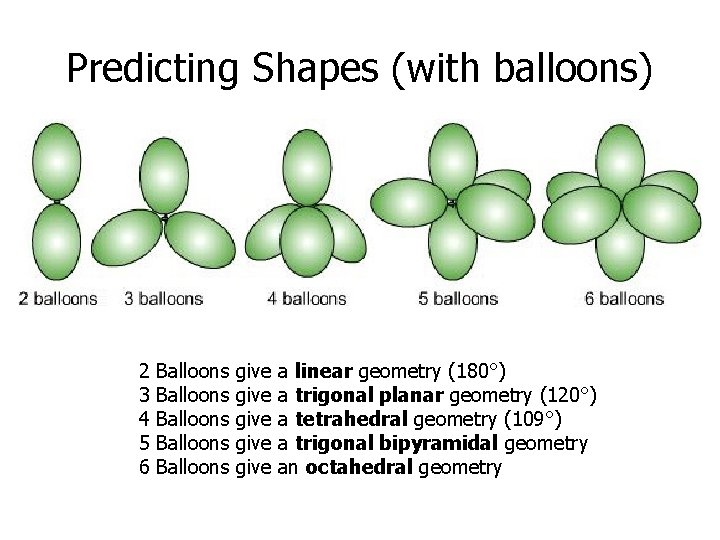
Predicting Shapes (with balloons) 2 3 4 5 6 Balloons Balloons give give a linear geometry (180°) a trigonal planar geometry (120°) a tetrahedral geometry (109°) a trigonal bipyramidal geometry an octahedral geometry
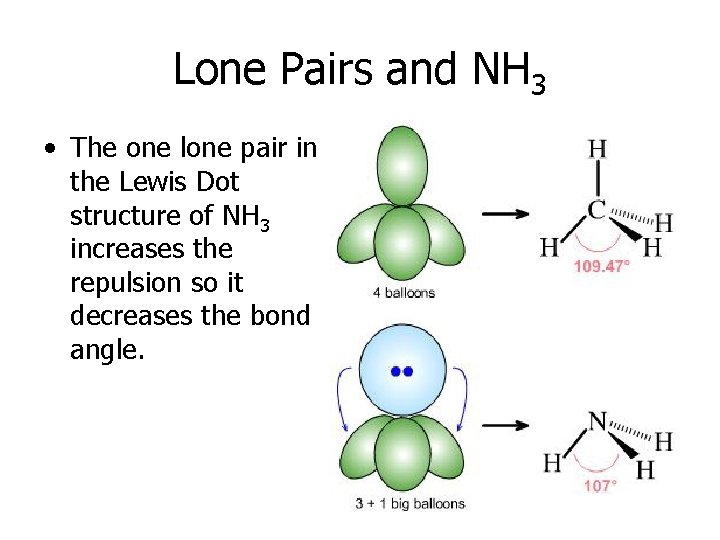
Lone Pairs and NH 3 • The one lone pair in the Lewis Dot structure of NH 3 increases the repulsion so it decreases the bond angle.
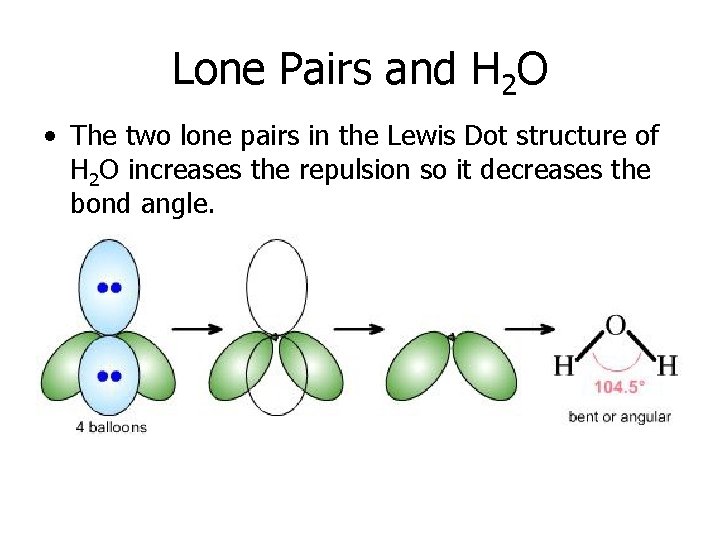
Lone Pairs and H 2 O • The two lone pairs in the Lewis Dot structure of H 2 O increases the repulsion so it decreases the bond angle.
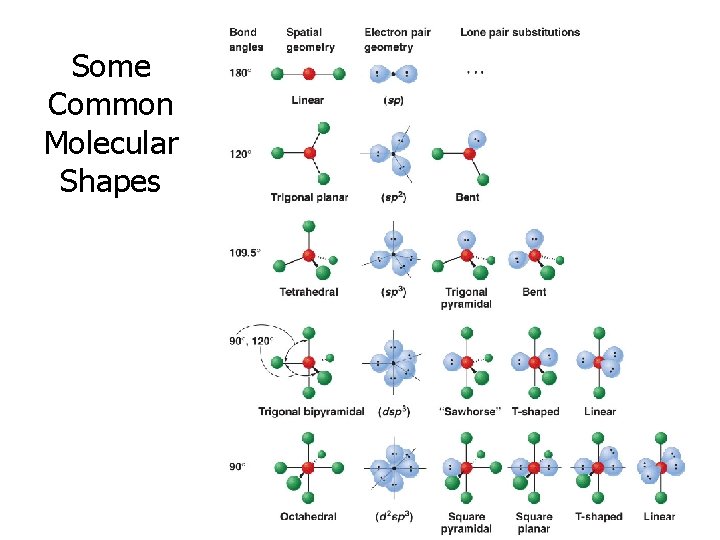
Some Common Molecular Shapes
Bonding Molecular Polarity
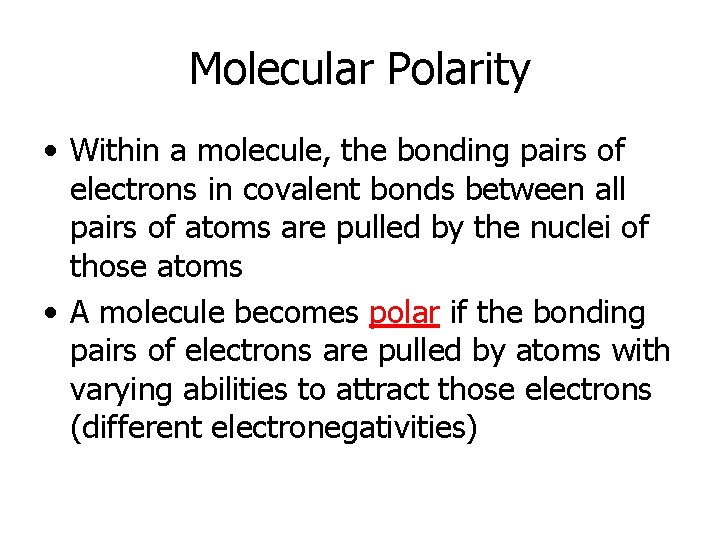
Molecular Polarity • Within a molecule, the bonding pairs of electrons in covalent bonds between all pairs of atoms are pulled by the nuclei of those atoms • A molecule becomes polar if the bonding pairs of electrons are pulled by atoms with varying abilities to attract those electrons (different electronegativities)
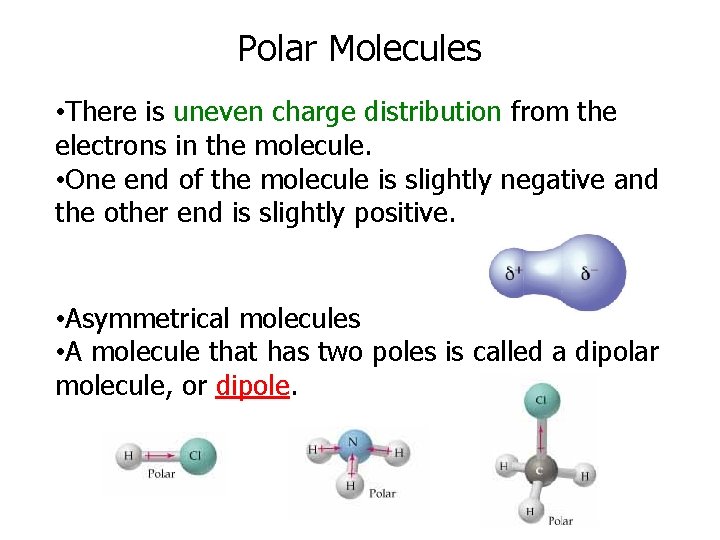
Polar Molecules • There is uneven charge distribution from the electrons in the molecule. • One end of the molecule is slightly negative and the other end is slightly positive. • Asymmetrical molecules • A molecule that has two poles is called a dipolar molecule, or dipole.
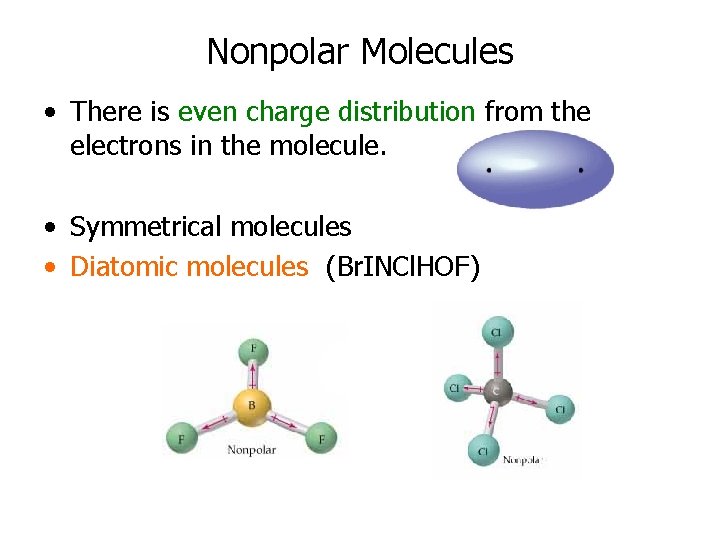
Nonpolar Molecules • There is even charge distribution from the electrons in the molecule. • Symmetrical molecules • Diatomic molecules (Br. INCl. HOF)
Bonding Metallic Bonding
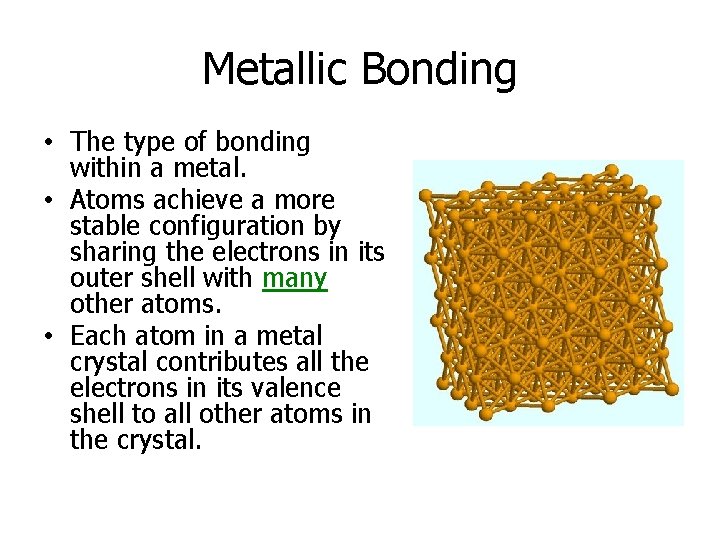
Metallic Bonding • The type of bonding within a metal. • Atoms achieve a more stable configuration by sharing the electrons in its outer shell with many other atoms. • Each atom in a metal crystal contributes all the electrons in its valence shell to all other atoms in the crystal.

Metallic Solids • Conduct electricity as a solid and a liquid. • Have metallic bonds between their atoms. • These special bonds have mobile valence electrons that are free to move.
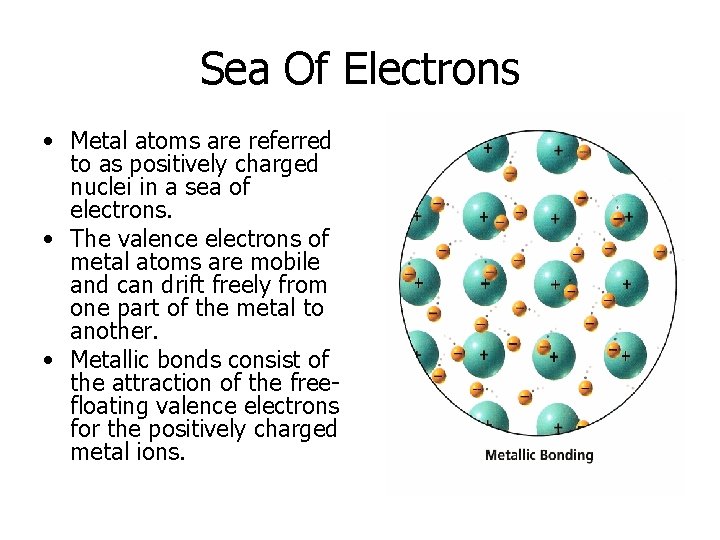
Sea Of Electrons • Metal atoms are referred to as positively charged nuclei in a sea of electrons. • The valence electrons of metal atoms are mobile and can drift freely from one part of the metal to another. • Metallic bonds consist of the attraction of the freefloating valence electrons for the positively charged metal ions.
Metallic Bonding • This explains why metals can “mix” together to form alloys (a homogeneous mixture of metals) • Examples: – Brass (Cu &Zn) – Sterling Silver (Ag & Cu) – Bronze (Cu, Zn, Ni, C) – Stainless Steel (Fe, Cr, C & Ni) – Cast Iron (Fe & C)
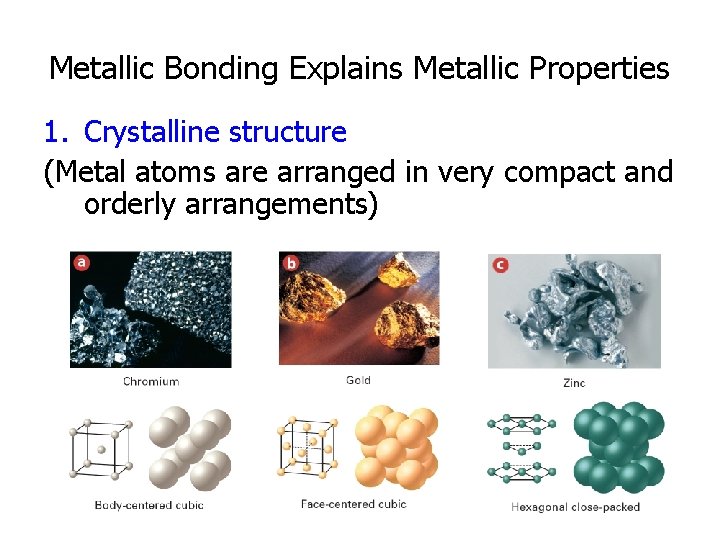
Metallic Bonding Explains Metallic Properties 1. Crystalline structure (Metal atoms are arranged in very compact and orderly arrangements)

2. Conductivity of heat & electricity (because electrons are flowing) 3. Hardness / durability (the electrons are “binding” the atoms together) 4. Luster (the way light is evenly absorbed & reflected by the electrons) 5. Ductility & Malleability (due to the uniform attraction between the positive nuclei and the negative electrons)

Why Are Alloys Important? • Their properties are often superior to those of their component elements. • The most important alloys today are steels. – Have a wide range of useful properties, such as: » » corrosion resistance ductility hardness toughness • Bicycle frames are often made of titanium alloys that contain aluminum and vanadium.

Bonding Intermolecular Forces of Attraction
Intermolecular Forces of Attraction • The attraction between molecules. • There are 3 types: 1. Van der Waals Forces (London Dispersion) 2. Dipole-dipole Interaction 3. Hydrogen Bonding
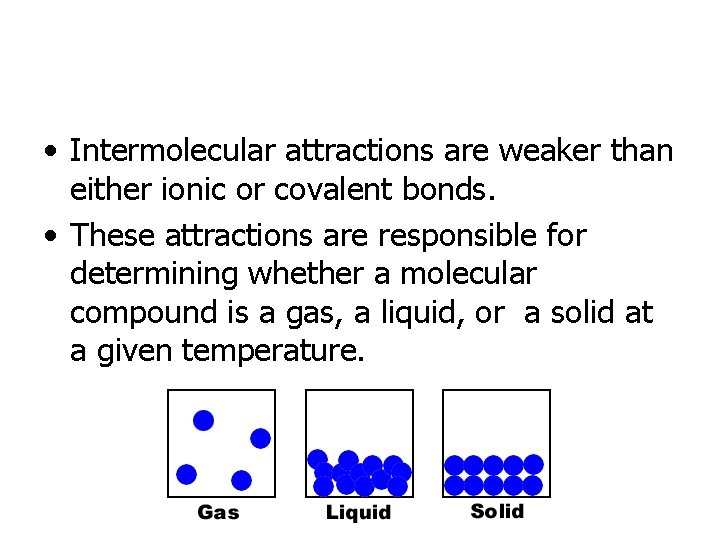
• Intermolecular attractions are weaker than either ionic or covalent bonds. • These attractions are responsible for determining whether a molecular compound is a gas, a liquid, or a solid at a given temperature.

Van der Waals Forces • Weakest force of attraction • It is the only force of attraction present between nonpolar molecules or between atoms in a noble gas • They are the result of temporary (or shifting) dipoles in molecules caused by the random, asymmetric motion of electrons • Explains why the Halogen group has gas, liquids, and solids • Force of attraction is directly related to the # of electrons

Van der Waals Force increases with: • • Lower temperature Higher pressure Increasing size of molecule/atom Decreasing distance between molecules Trend: • As you go down a group on the periodic table, atomic radius increases, therefore the size of the atom/moleucle increases and thus the forces between the atom(s)/molecule(s) increases.
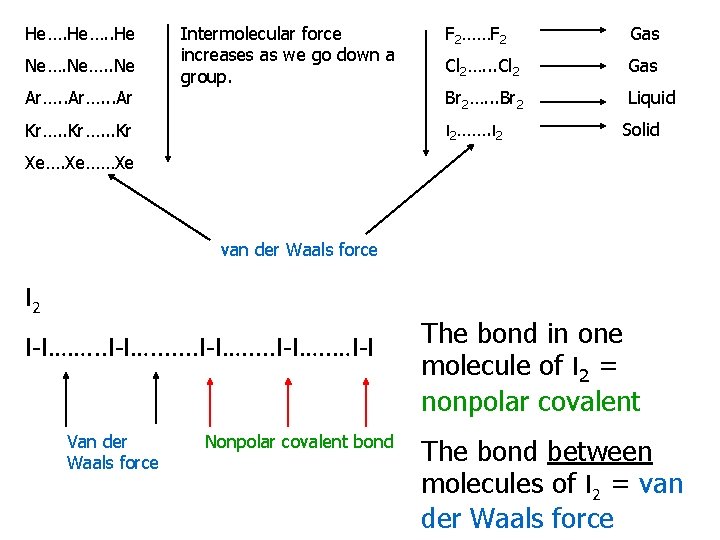
He…. . He Ne…. . Ne Ar…. . . Ar Intermolecular force increases as we go down a group. F 2……F 2 Gas Cl 2…. . . Cl 2 Gas Br 2…. . . Br 2 Liquid I 2……. I 2 Kr…. . . Kr Solid Xe……Xe van der Waals force I 2 I-I……. . . I-I…. . …I-I Van der Waals force Nonpolar covalent bond The bond in one molecule of I 2 = nonpolar covalent The bond between molecules of I 2 = van der Waals force
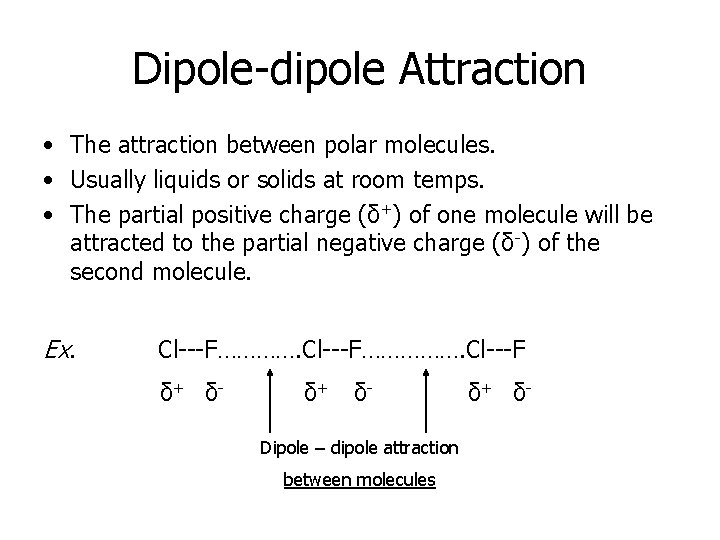
Dipole-dipole Attraction • The attraction between polar molecules. • Usually liquids or solids at room temps. • The partial positive charge (δ+) of one molecule will be attracted to the partial negative charge (δ-) of the second molecule. Ex. Cl---F……………. Cl---F δ+ δ- Dipole – dipole attraction between molecules δ+ δ-
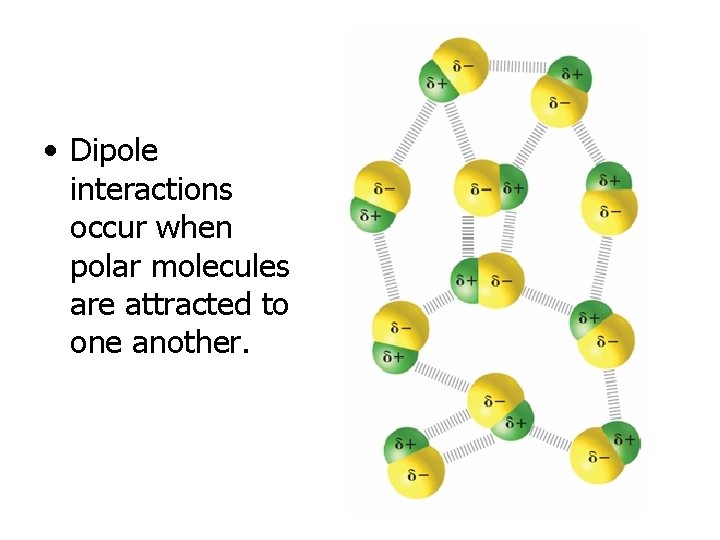
• Dipole interactions occur when polar molecules are attracted to one another.
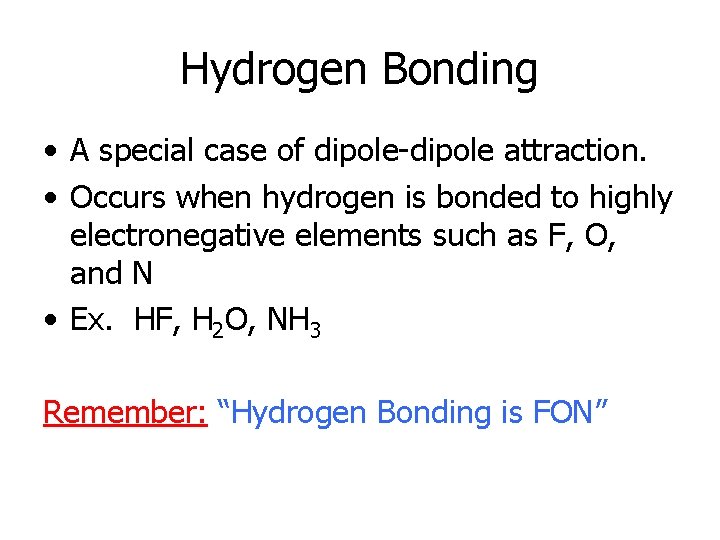
Hydrogen Bonding • A special case of dipole-dipole attraction. • Occurs when hydrogen is bonded to highly electronegative elements such as F, O, and N • Ex. HF, H 2 O, NH 3 Remember: “Hydrogen Bonding is FON”
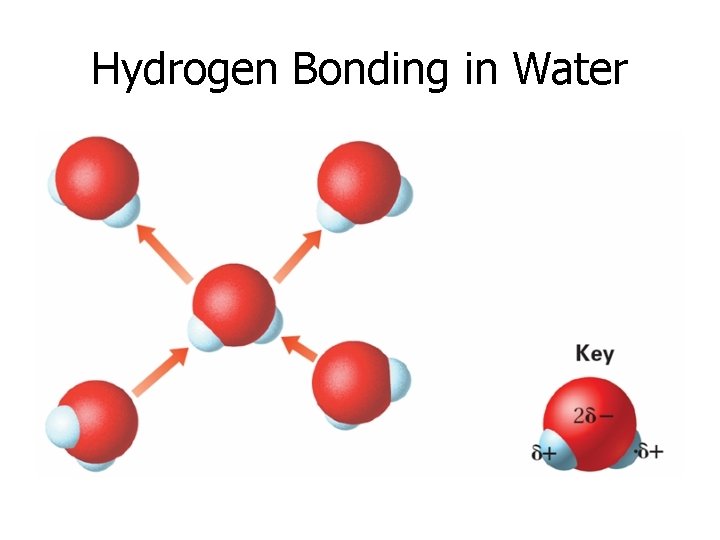
Hydrogen Bonding in Water
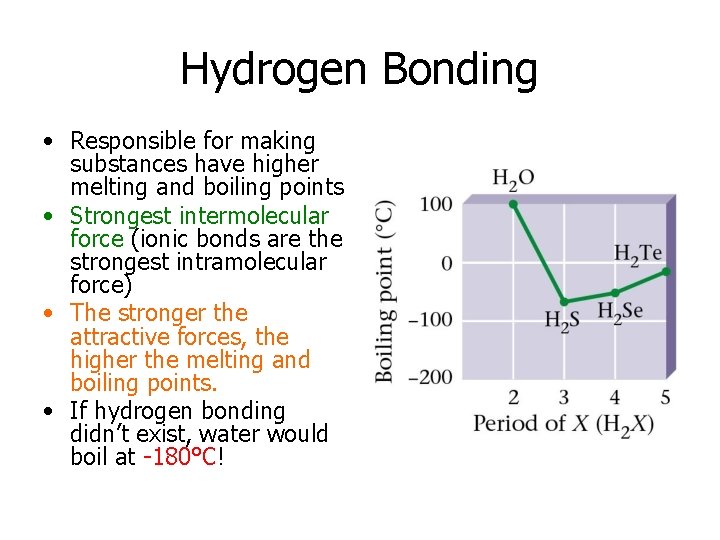
Hydrogen Bonding • Responsible for making substances have higher melting and boiling points • Strongest intermolecular force (ionic bonds are the strongest intramolecular force) • The stronger the attractive forces, the higher the melting and boiling points. • If hydrogen bonding didn’t exist, water would boil at -180°C!
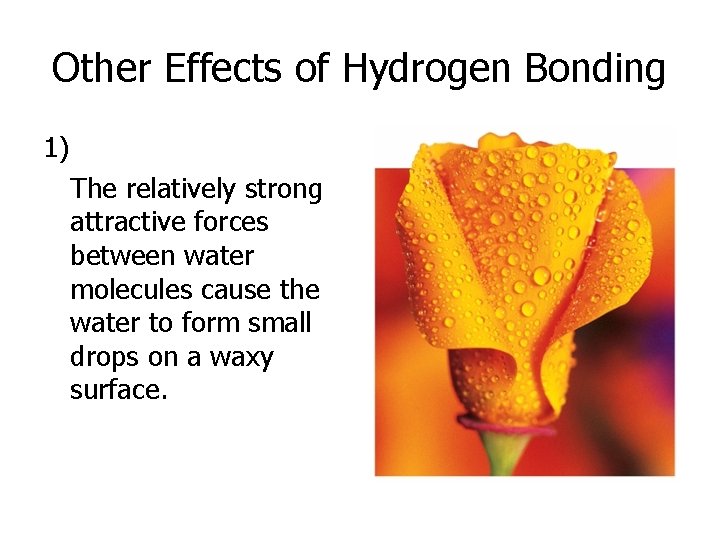
Other Effects of Hydrogen Bonding 1) The relatively strong attractive forces between water molecules cause the water to form small drops on a waxy surface.

2) Has an effect on the crystal structure of ice – Allows for the open space in the middle of the hexagonal ice structure – Explains why ice floats in water
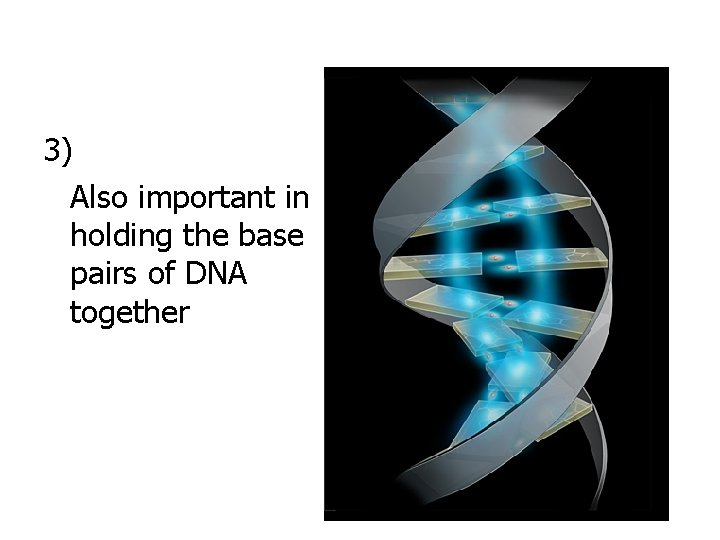
3) Also important in holding the base pairs of DNA together
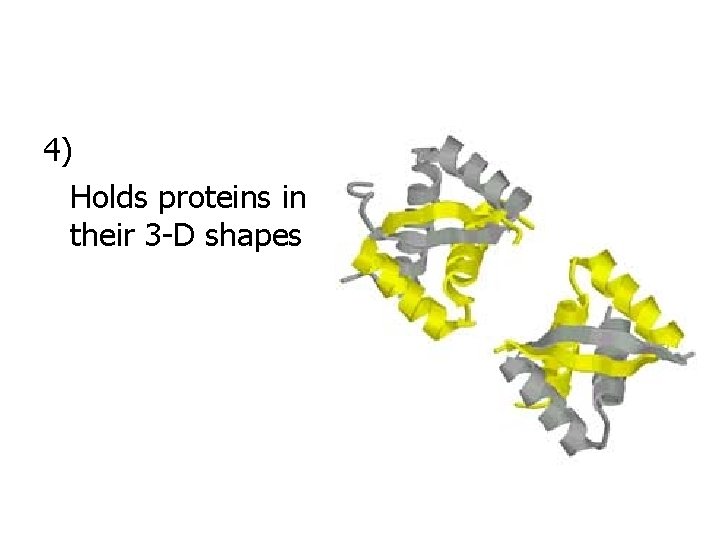
4) Holds proteins in their 3 -D shapes
- Slides: 73