Bonding 1 What two types of bonds form
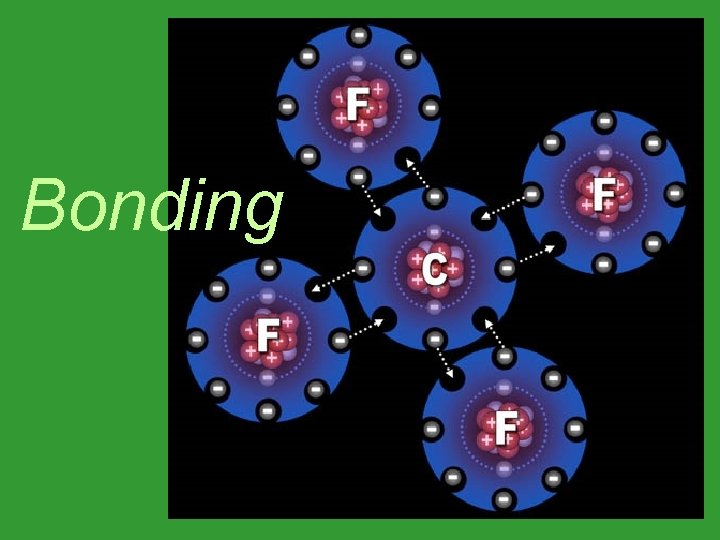
Bonding

1. What two types of bonds form and how do they form?
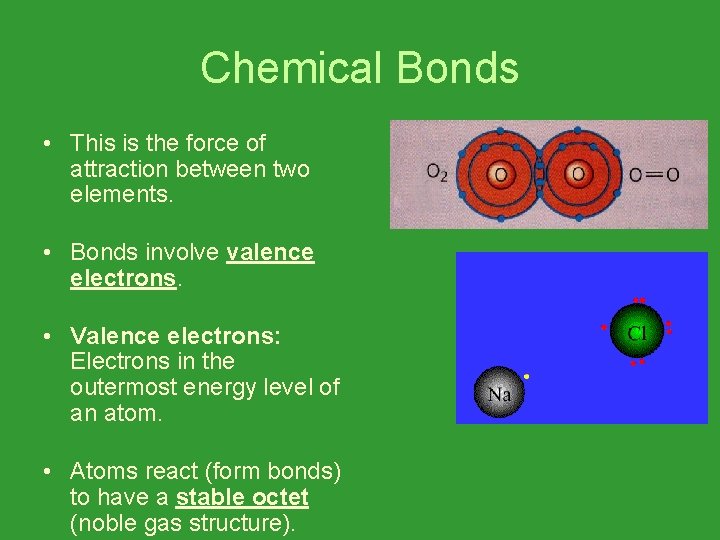
Chemical Bonds • This is the force of attraction between two elements. • Bonds involve valence electrons. • Valence electrons: Electrons in the outermost energy level of an atom. • Atoms react (form bonds) to have a stable octet (noble gas structure).

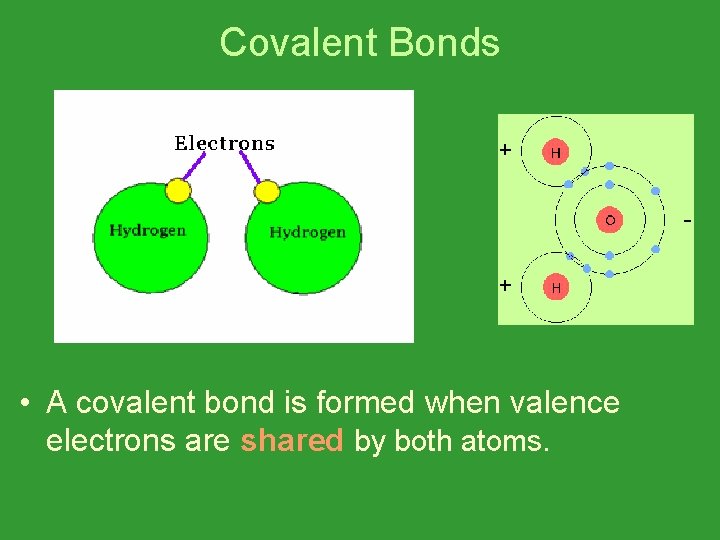
Covalent Bonds • A covalent bond is formed when valence electrons are shared by both atoms.
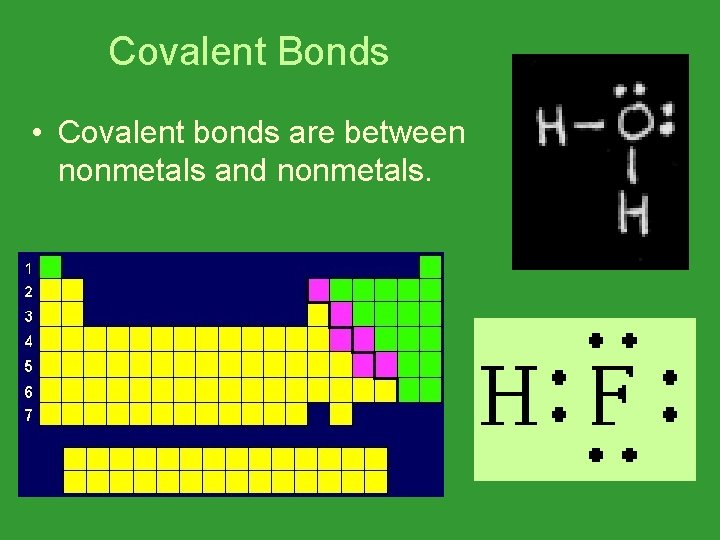
Covalent Bonds • Covalent bonds are between nonmetals and nonmetals.
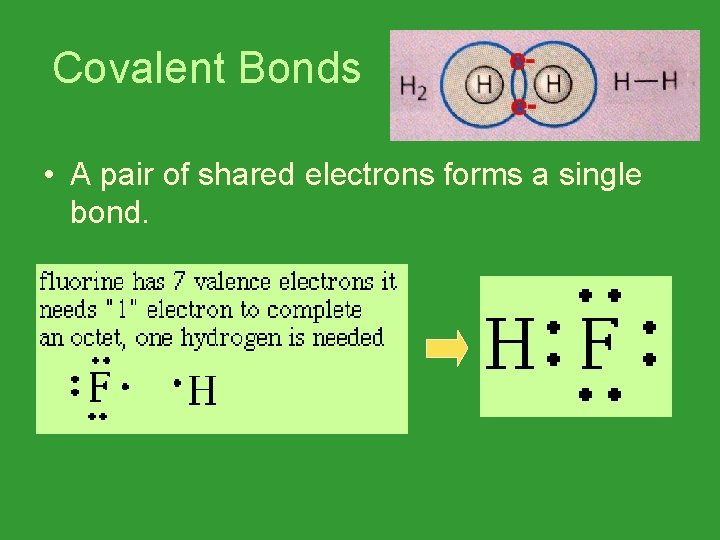
Covalent Bonds • A pair of shared electrons forms a single bond.
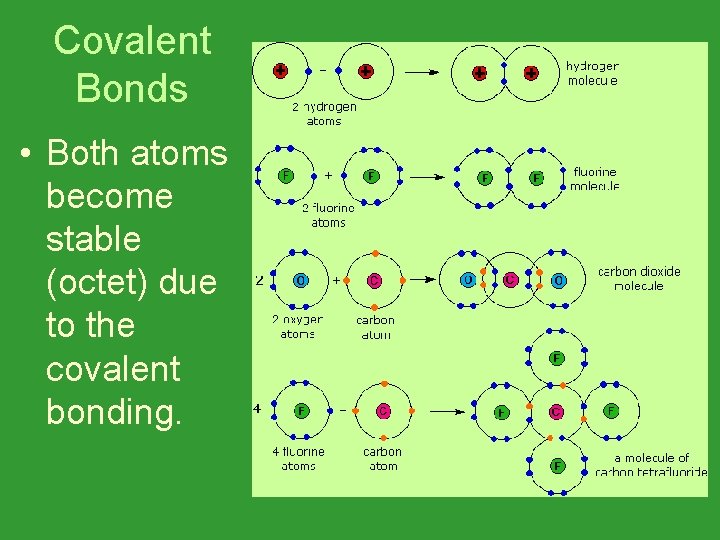
Covalent Bonds • Both atoms become stable (octet) due to the covalent bonding.
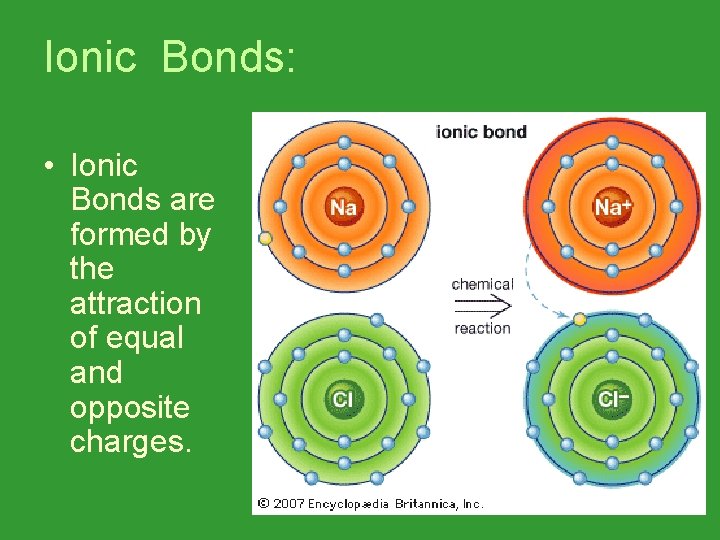
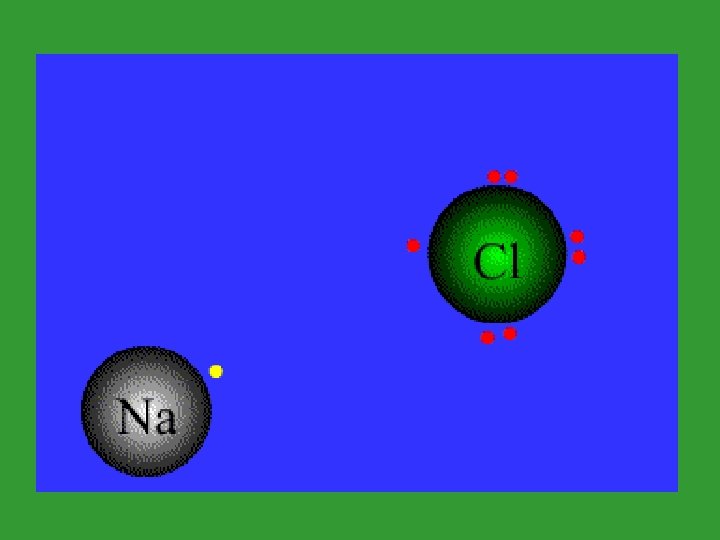
Ionic Bonds: • Ionic Bonds are formed by the attraction of equal and opposite charges.

Ionic Bonds • Electrons are transferred in an ionic bond.
Ionic Bonds
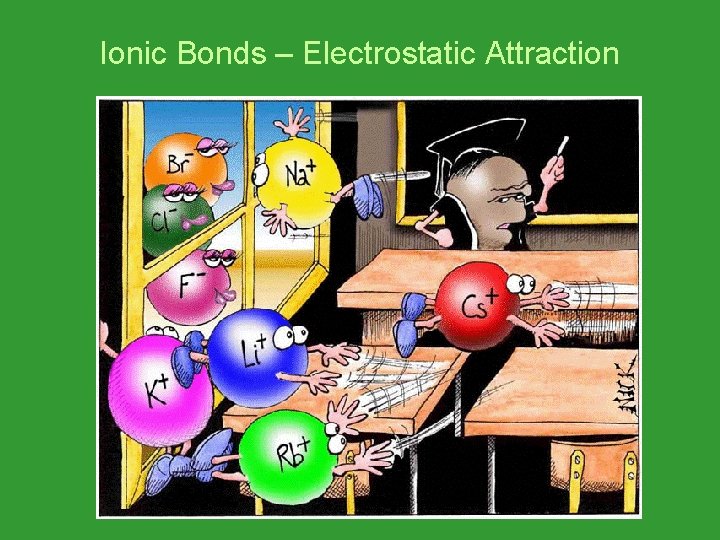
Ionic Bonds – Electrostatic Attraction
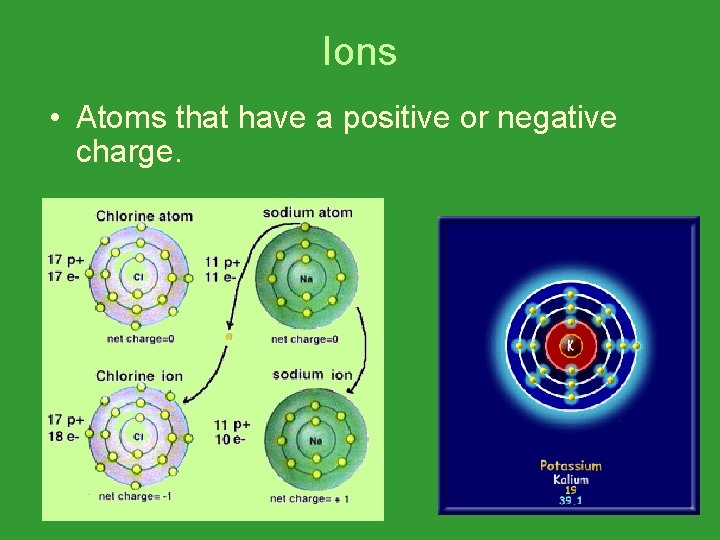
Ions • Atoms that have a positive or negative charge.
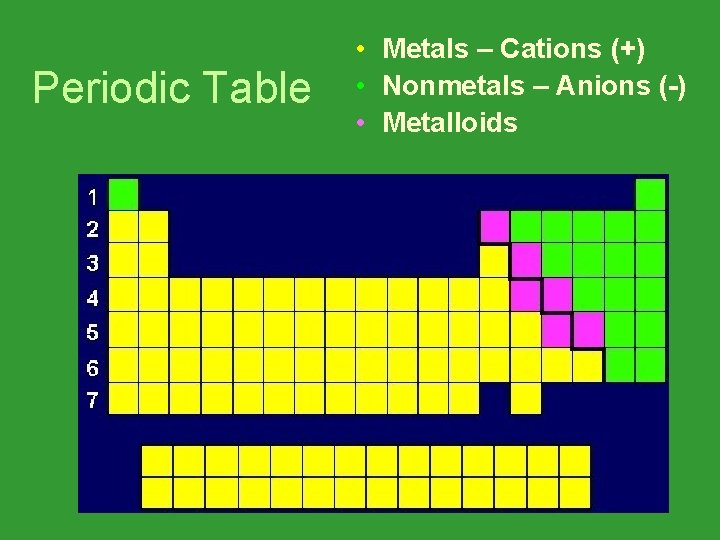
Periodic Table • Metals – Cations (+) • Nonmetals – Anions (-) • Metalloids
Ionic Bonds occur between: 1. A metal (cation) and a nonmetal (anion) 2. A metal (cation) and a negative polyatomic ion 3. A positive polyatomic ion and a nonmetal (anion) 4. A positive polyatomic ion and a negative polyatomic ion
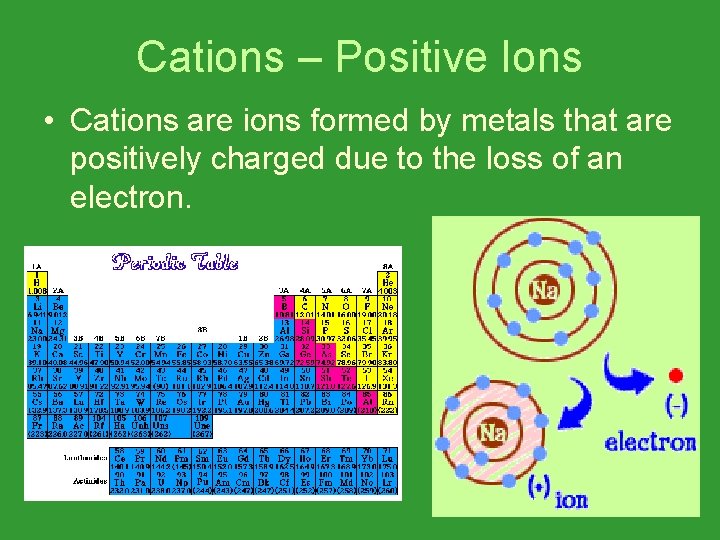
Cations – Positive Ions • Cations are ions formed by metals that are positively charged due to the loss of an electron.

Cations – Positive Ions • Cations have low ionization energy. • They lose electrons easily to become positive.
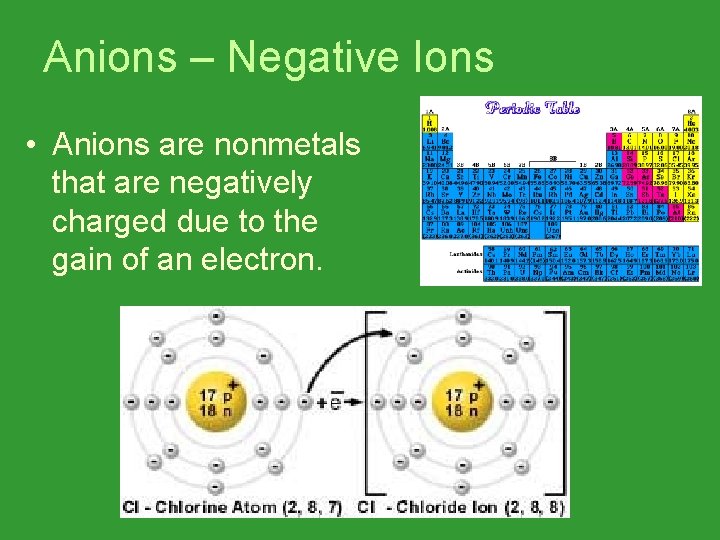
Anions – Negative Ions • Anions are nonmetals that are negatively charged due to the gain of an electron.
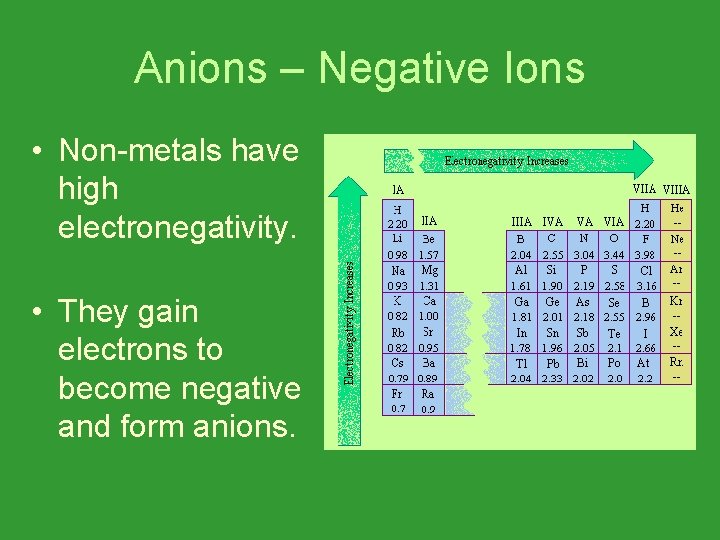
Anions – Negative Ions • Non-metals have high electronegativity. • They gain electrons to become negative and form anions.
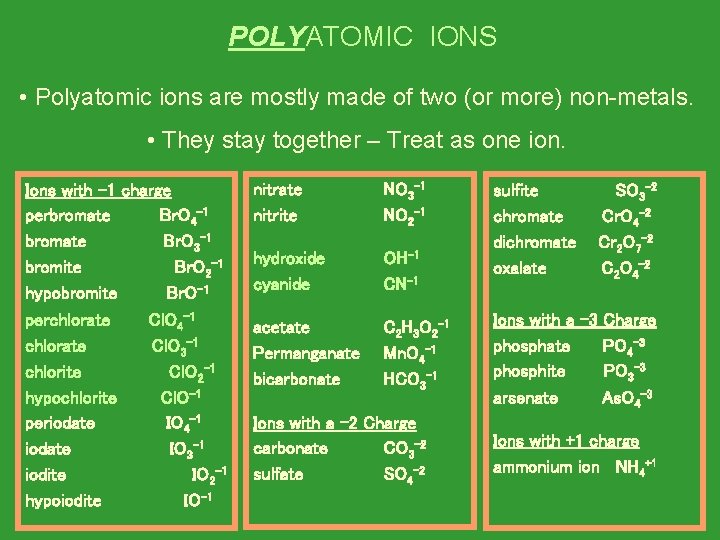
POLYATOMIC IONS • Polyatomic ions are mostly made of two (or more) non-metals. • They stay together – Treat as one ion. Ions with -1 charge perbromate Br. O 4 -1 bromate Br. O 3 -1 bromite Br. O 2 -1 hypobromite Br. O-1 perchlorate Cl. O 4 -1 chlorate Cl. O 3 -1 chlorite Cl. O 2 -1 hypochlorite Cl. O-1 periodate IO 4 -1 iodate IO 3 -1 iodite IO 2 -1 hypoiodite IO-1 nitrate nitrite NO 3 -1 NO 2 -1 hydroxide cyanide OH-1 CN-1 acetate Permanganate bicarbonate C 2 H 3 O 2 -1 Mn. O 4 -1 HCO 3 -1 Ions with a -2 Charge carbonate CO 3 -2 sulfate SO 4 -2 sulfite chromate dichromate oxalate SO 3 -2 Cr. O 4 -2 Cr 2 O 7 -2 C 2 O 4 -2 Ions with a -3 Charge phosphate PO 4 -3 phosphite PO 3 -3 arsenate As. O 4 -3 Ions with +1 charge ammonium ion NH 4+1
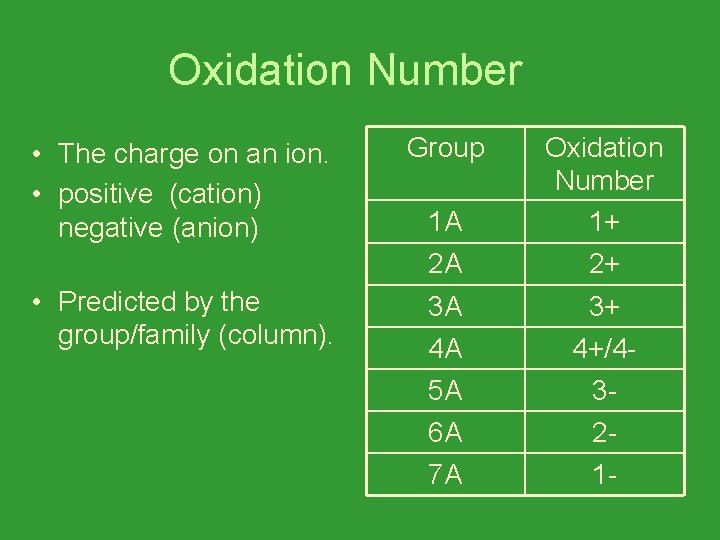
Oxidation Number • The charge on an ion. • positive (cation) negative (anion) • Predicted by the group/family (column). Group 1 A Oxidation Number 1+ 2 A 2+ 3 A 3+ 4 A 4+/4 - 5 A 3 - 6 A 2 - 7 A 1 -
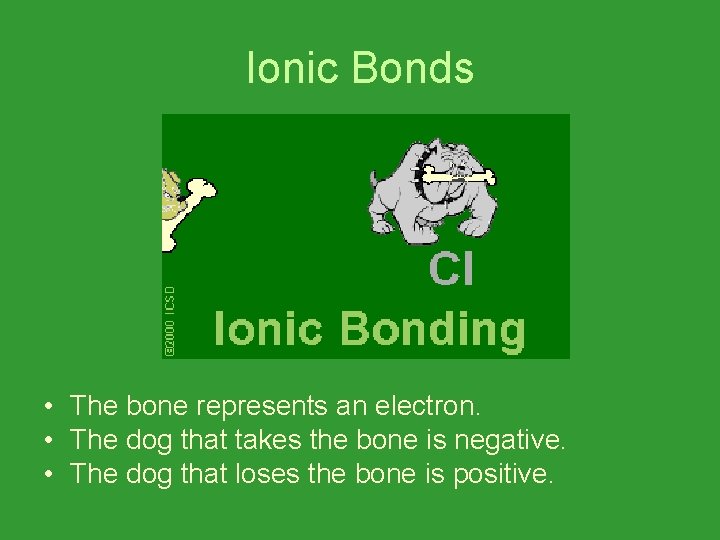
Ionic Bonds • The bone represents an electron. • The dog that takes the bone is negative. • The dog that loses the bone is positive.
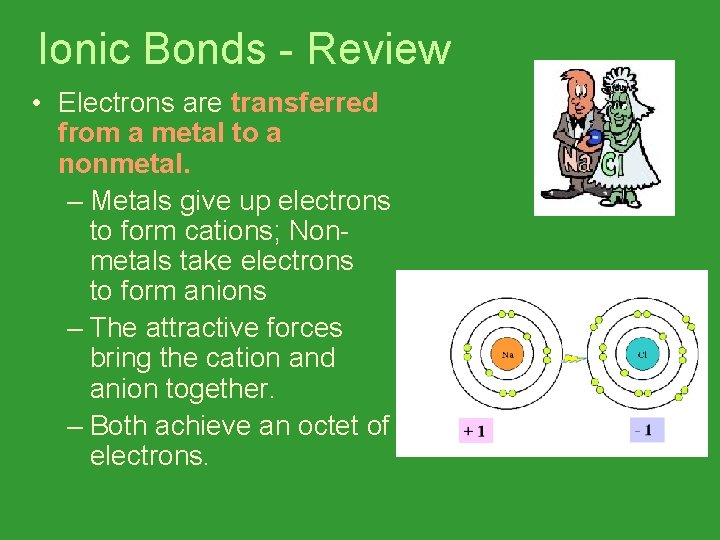
Ionic Bonds - Review • Electrons are transferred from a metal to a nonmetal. – Metals give up electrons to form cations; Nonmetals take electrons to form anions – The attractive forces bring the cation and anion together. – Both achieve an octet of electrons.
Ionic Bonds Quiz • 1) In an ionic bond, electrons are _____. • 2) The type of electrons involved are ___ or outer shell electrons. • 3) Metals ___ electrons, nonmetals ___ electrons in order to get a ___ gas configuration (stable octet). • 4) Cations have a __ charge and are formed by __. • 5) Anions have a __ charge and are formed by __. • 6) ____ charges hold the compound together.

2. How are ionic compounds formed?


Ionic Bonds Essential Question #2: See Essential Question #1

3. How are ionic compounds named?

Naming Binary Ionic Compounds v The cation (+ ion) is written first, followed by the anion (- ion). v Atoms combine so that they are neutral. 1. Name 1 st element as is (cation). 2. Name 2 nd element (anion) using the “ide” ending. • What is the name of Al. Br 3? • Aluminum bromide

Practice Naming Ionic Compounds • • • 1) Na. Cl Sodium chloride 2) Mg. F 2 Magnesium fluoride 3) Al 2 O 3 Aluminum oxide • • • 4) Ca. S Calcium sulfide 5) Ga. P Gallium phosphide 6) K 2 O Potassium oxide

NAMING TERNARY Ionic COMPOUNDS (POLYATOMIC IONS) • 1) Name cation (+) ion. • 2) Name the polyatomic ion as a group. (ate, ite) • Ex. Ca. SO 4 • • • cation=calcium anion=sulfate Name: Calcium Sulfate

Practice: Name the Compound • 1) Na. Cl. O 3 • sodium chlorate • 2) Al. PO 4 • aluminum phosphate • 3) (NH 4)2 S • ammonium sulfide • 5) Ca. Cl 2 calcium chloride

Writing Formulas for Ionic Compounds 1. The oxidation numbers have to add up to zero. 2. Determine oxidation numbers of the ions. 3. Cations – Look at Periodic Table. 4. Anions – Look at the Periodic Table or Polyatomic Ion chart. 5. Balance the charges by adding subscripts. 6. Put polyatomic ions in parenthesis if there is more than one.

Writing Formulas for Ionic Compounds • Write the formula for calcium chloride. • Calcium is Ca 2+ • Chloride is Cl 1 • Ca 2+ Cl 1 - would have a 1+ charge. • Need another Cl 1 • Ca 2+ Cl 21 -
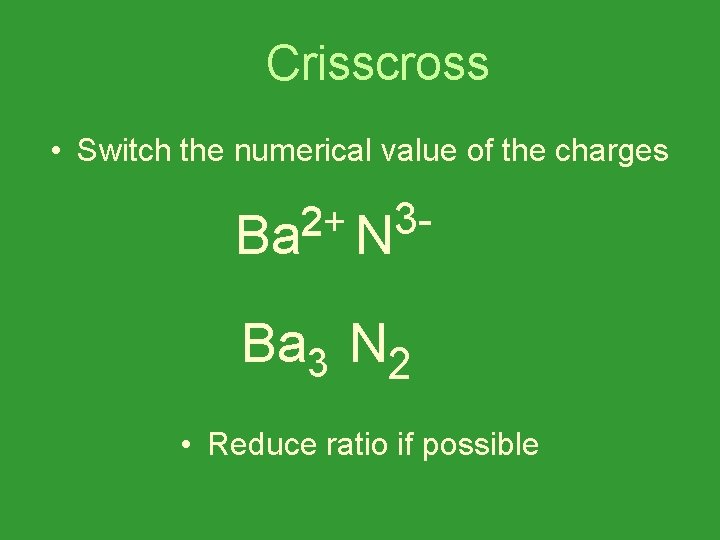
Crisscross • Switch the numerical value of the charges 3 32 2+ Ba N Ba 3 N 2 • Reduce ratio if possible
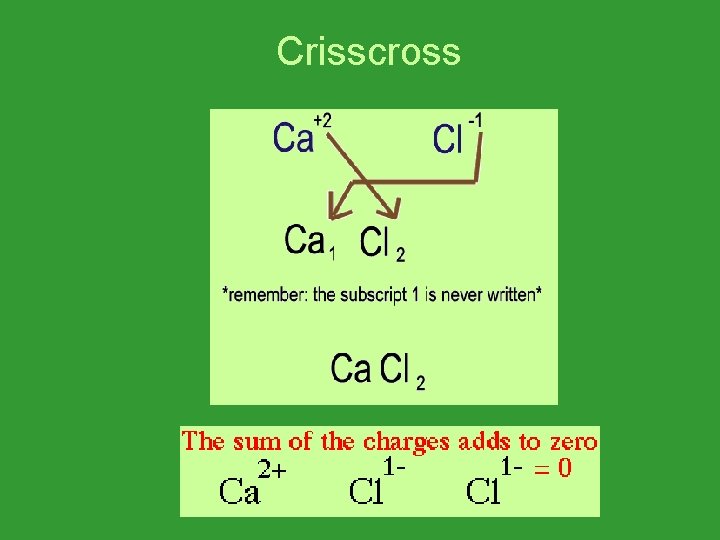
Crisscross

Practice Writing Ionic Formulas Aluminum Bromide • cation anion • Al 3+ Br • Al. Br 3 Sodium Oxide • Na+ O 2 • Na 2 O
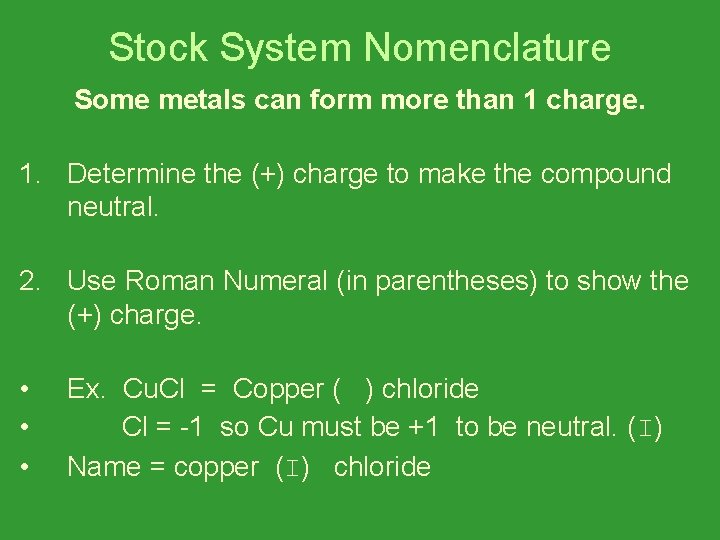
Stock System Nomenclature Some metals can form more than 1 charge. 1. Determine the (+) charge to make the compound neutral. 2. Use Roman Numeral (in parentheses) to show the (+) charge. • • • Ex. Cu. Cl = Copper ( ) chloride Cl = -1 so Cu must be +1 to be neutral. (I) Name = copper (I) chloride
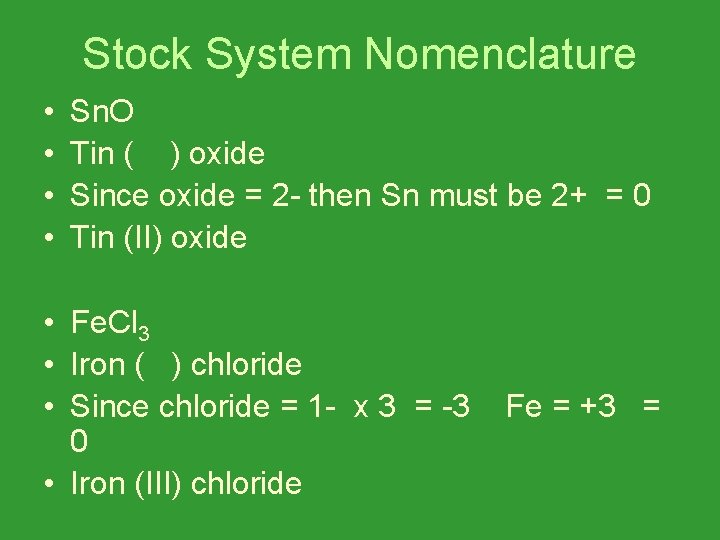
Stock System Nomenclature • • Sn. O Tin ( ) oxide Since oxide = 2 - then Sn must be 2+ = 0 Tin (II) oxide • Fe. Cl 3 • Iron ( ) chloride • Since chloride = 1 - x 3 = -3 0 • Iron (III) chloride Fe = +3 =
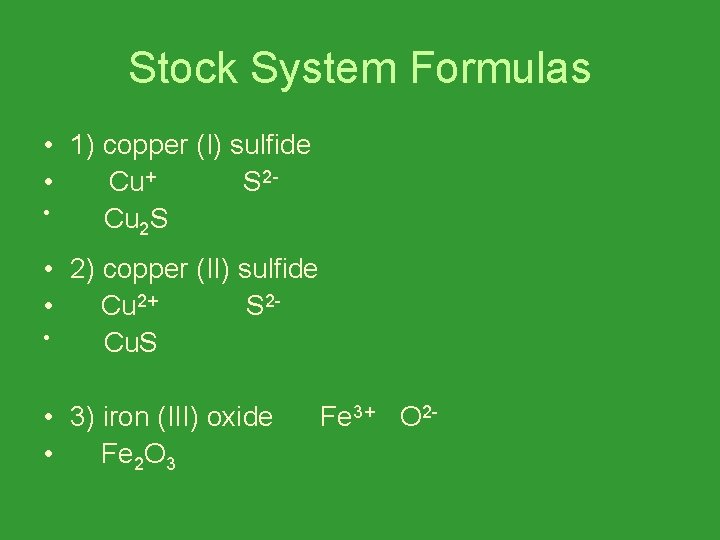
Stock System Formulas • 1) copper (I) sulfide • Cu+ S 2 • Cu 2 S • 2) copper (II) sulfide • Cu 2+ S 2 • Cu. S • 3) iron (III) oxide • Fe 2 O 3 Fe 3+ O 2 -

Name the Compounds * Use Stock System* • 1) * Cu. Cl • Cl = -1 Cu = +1 Copper (I) chloride • 2) * Cu. Cl • • • Cl = -1 x 2 = -2 3) * Pb. S S = -2 Pb = +2 4) * Pb. S 2 S = -2 x 2 = -4 Pb=+4 5) * Fe. Br 3 Br = -1 x 3 = -3 Fe=+3 6) Ag. I Ag has only 1 ion, +1 Copper (II) chloride Lead (II) sulfide Lead (IV) sulfide Iron (III) bromide Silver iodide

Choose covalent (2 nonmetals) or ionic (metal+nonmetal or polyatomic). Determine Names. 1. 2. 3. 4. 5. 6. KCl NO Na. NO 3 NH 4 Cl SO 3 CBr 4
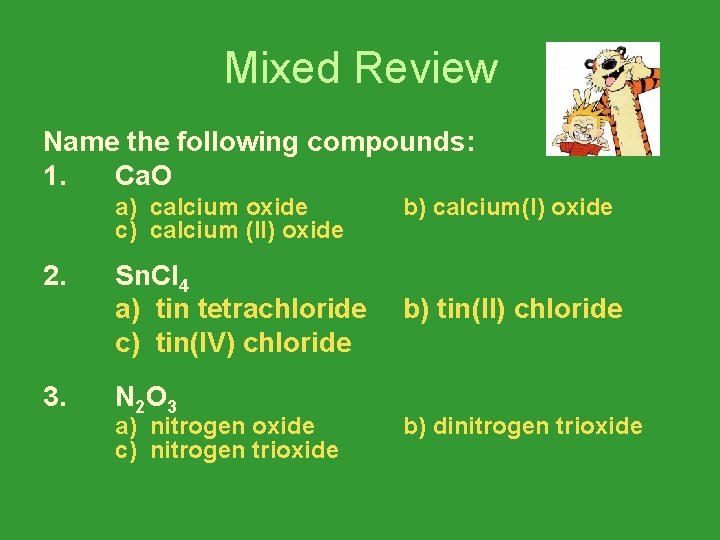
Mixed Review Name the following compounds: 1. Ca. O 2. 3. a) calcium oxide c) calcium (II) oxide b) calcium(I) oxide Sn. Cl 4 a) tin tetrachloride c) tin(IV) chloride b) tin(II) chloride N 2 O 3 a) nitrogen oxide c) nitrogen trioxide b) dinitrogen trioxide

Mixed Review 1. 2. 3. 4. 5. 6. 7. 8. 9. Dinitrogen monoxide Potassium sulfide Copper (II) nitrate Dichlorine heptoxide Chromium (III) sulfate Iron (III) sulfite Calcium oxide Barium carbonate Iodine monochloride

4. What is unique about ionic compounds that have Hydrogen as a cation?

Acids
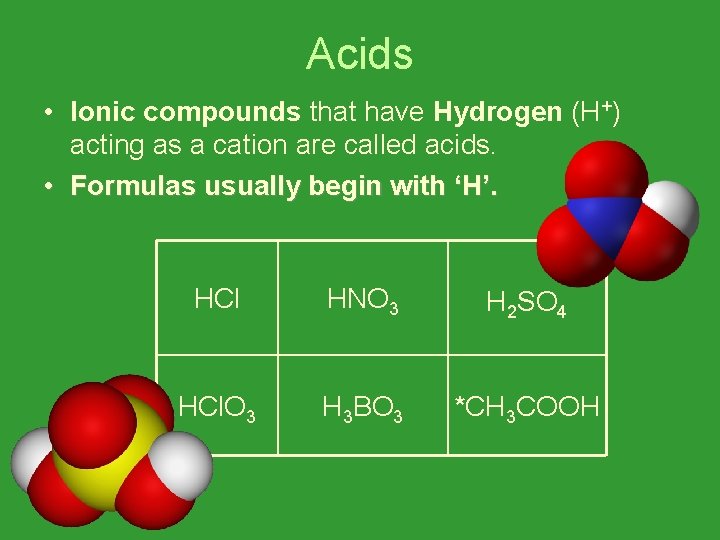
Acids • Ionic compounds that have Hydrogen (H+) acting as a cation are called acids. • Formulas usually begin with ‘H’. HCl HNO 3 H 2 SO 4 HCl. O 3 H 3 BO 3 *CH 3 COOH

5. How are acids named?
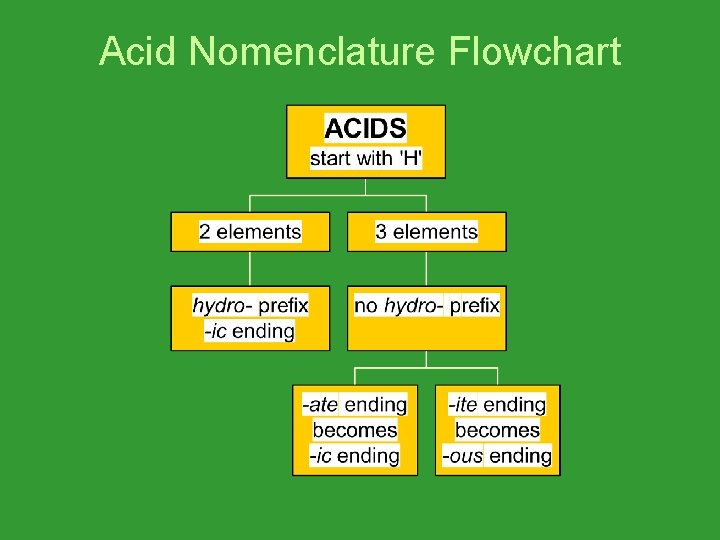
Acid Nomenclature Flowchart

How to Name Binary Acids: • hydro_____ic acid. • Example: HCl • - Hydrochloric Acid • Example: HF • - Hydrofluoric Acid
How to Name Tertiary Acids: • Tertiary acids ending in –ate: ______ ic acid • Tertiary acids ending in –ite: ______ous acid • An easy way to remember which goes with which… “In the cafeteria, you ATE something ICky”
How to Name Tertiary Acids: Examples • H 2 SO 4 • sulfuric acid • HNO 2 • nitrous acid

6. How are covalent compounds formed?

Covalent Bonds Essential Question #6: See Essential Question #1

7. What are the two types of covalent compounds that can be formed based on the way electrons are shared?
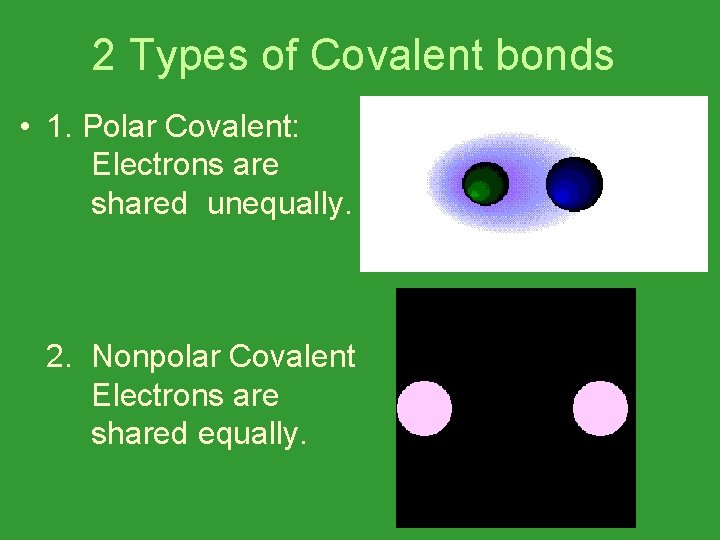
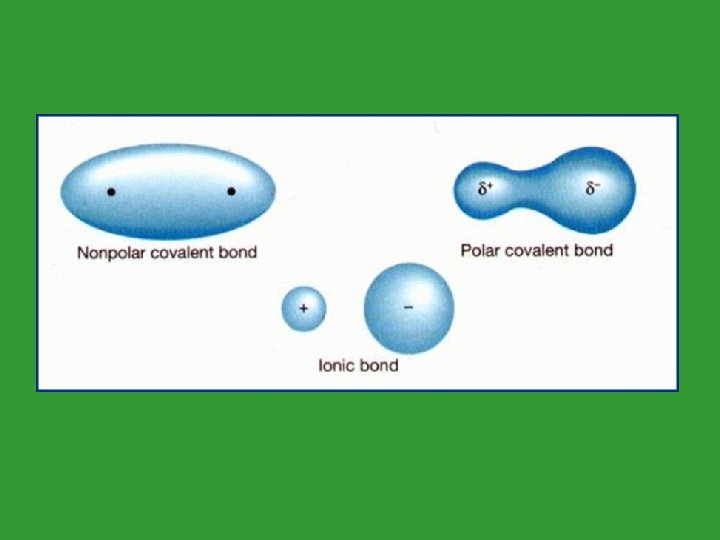
2 Types of Covalent bonds • 1. Polar Covalent: Electrons are shared unequally. 2. Nonpolar Covalent Electrons are shared equally.
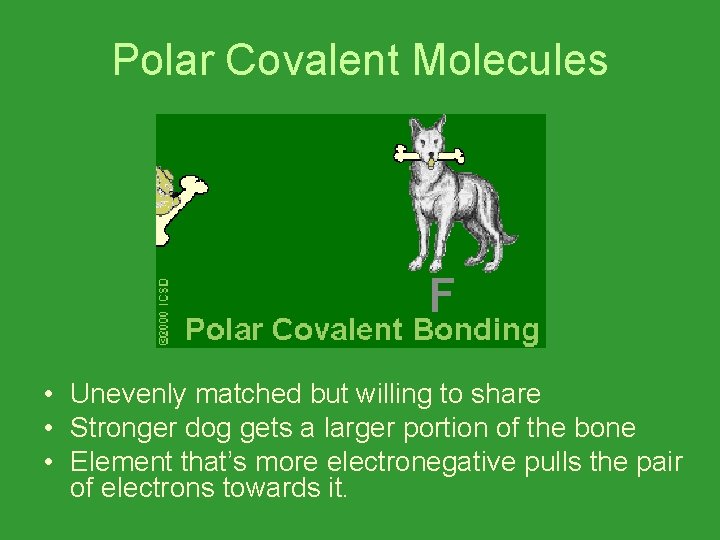
Polar Covalent Molecules • Unevenly matched but willing to share • Stronger dog gets a larger portion of the bone • Element that’s more electronegative pulls the pair of electrons towards it.
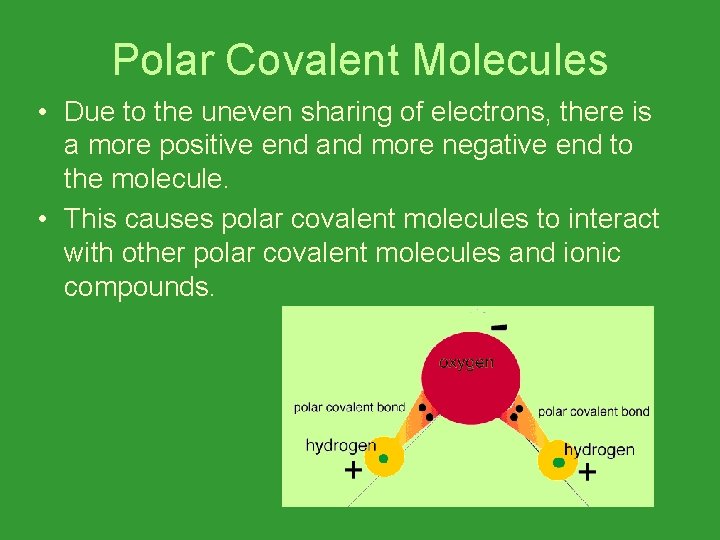
Polar Covalent Molecules • Due to the uneven sharing of electrons, there is a more positive end and more negative end to the molecule. • This causes polar covalent molecules to interact with other polar covalent molecules and ionic compounds.
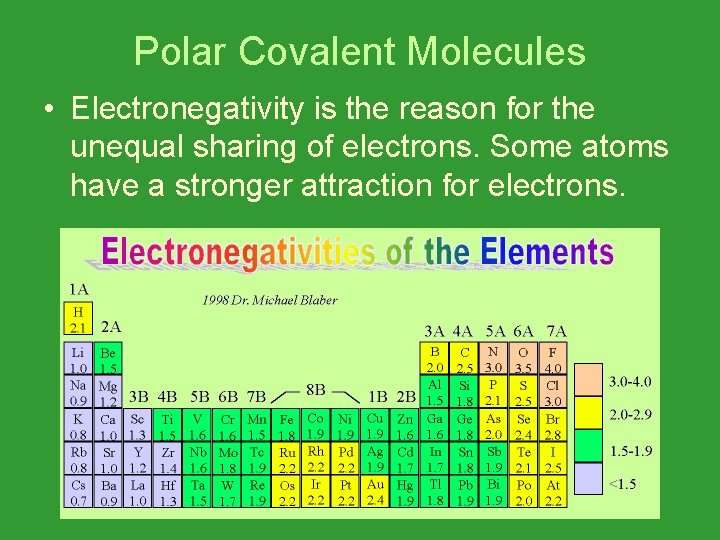
Polar Covalent Molecules • Electronegativity is the reason for the unequal sharing of electrons. Some atoms have a stronger attraction for electrons.
Nonpolar Covalent Molecules • Dogs of equal strength • Both dogs have equal attraction for the bone
Nonpolar Covalent Molecules • There are no partial charges. • Nonpolar Covalent Molecules only dissolve in other nonpolar covalent molecules.

8. How are covalent compounds named?
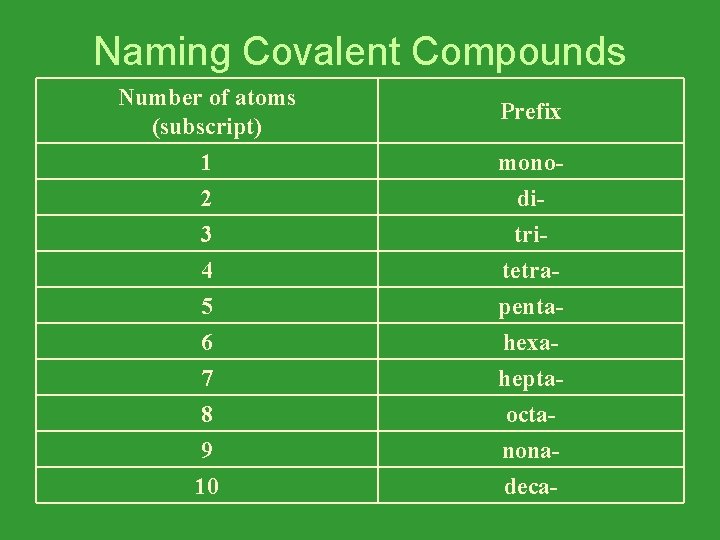
Naming Covalent Compounds Number of atoms (subscript) 1 2 monodi- 3 4 5 6 7 8 9 10 tritetrapentahexaheptaoctanonadeca- Prefix

Covalent Bond Naming Rules • Use Prefixes to indicate the number of atoms of each element (Look at the subscripts). • Add the suffix “–ide” to the ending of the name of the last element. • Remember that “mono” is not used for the first element, only subsequent elements.
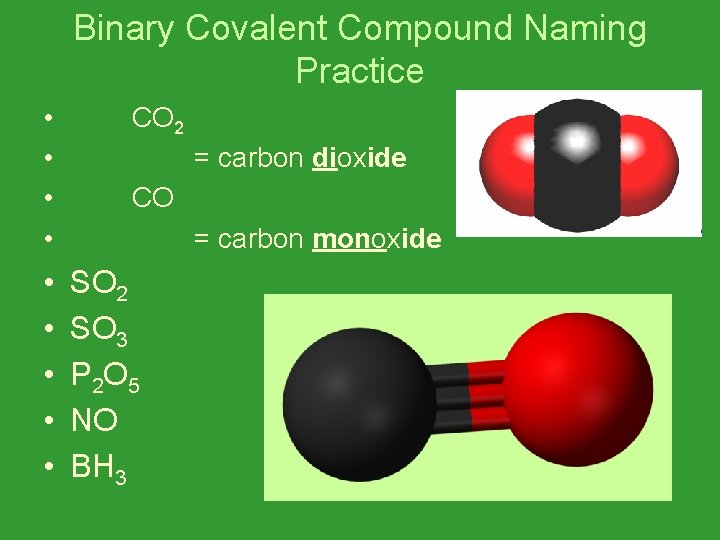
Binary Covalent Compound Naming Practice • • • CO 2 = carbon dioxide CO = carbon monoxide SO 2 SO 3 P 2 O 5 NO BH 3
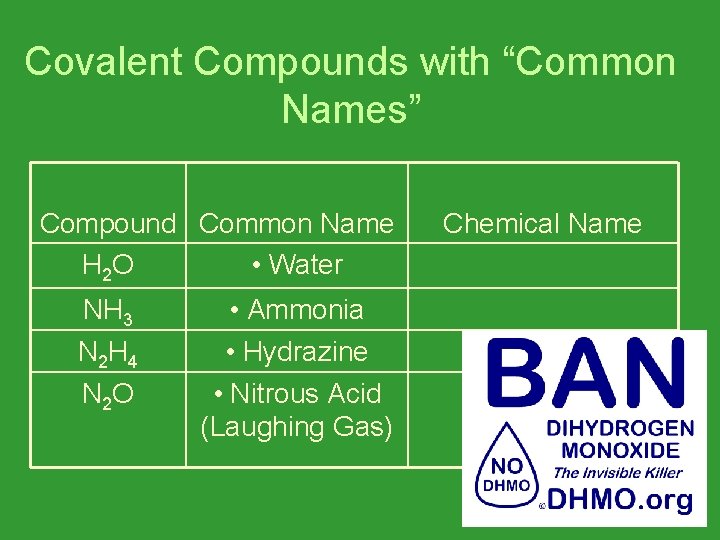
Covalent Compounds with “Common Names” Compound Common Name H 2 O • Water NH 3 N 2 H 4 N 2 O • Ammonia • Hydrazine • Nitrous Acid (Laughing Gas) Chemical Name

9. What are organic compounds and how are they named?
Organic Compounds • These are carbon-containing compounds made of hydrocarbons and their derivitives. • Organic compounds are covalently bonded. • They are often produced by living things. • Carbon is able to bond and form many different compounds.
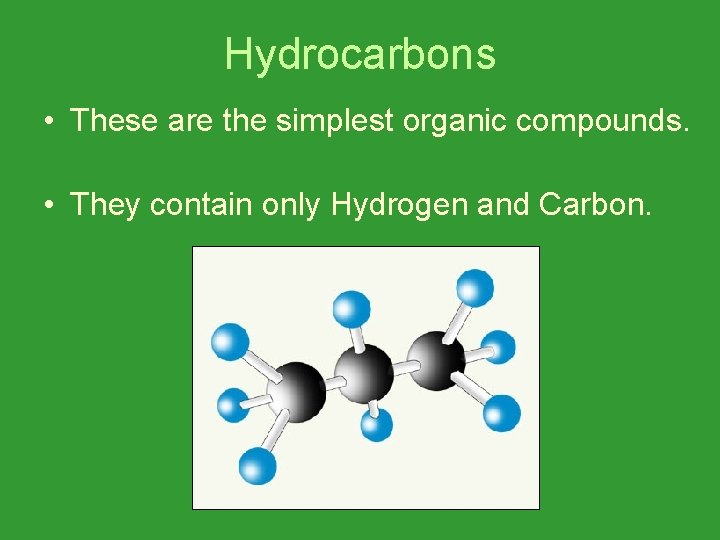
Hydrocarbons • These are the simplest organic compounds. • They contain only Hydrogen and Carbon.
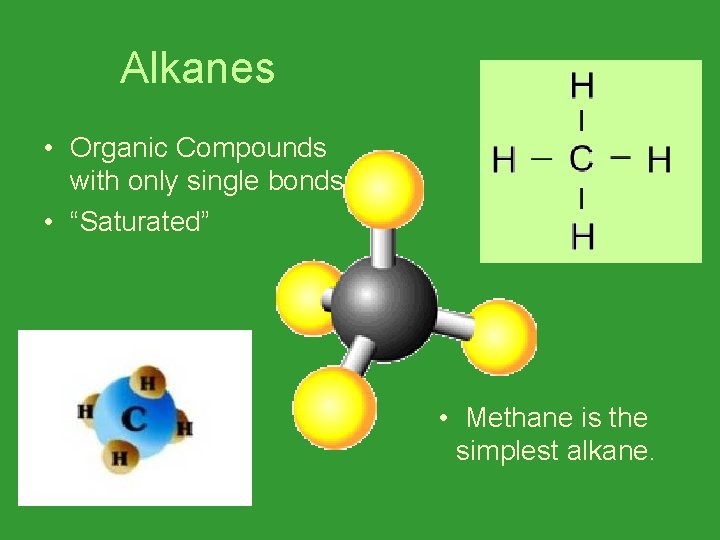
Alkanes • Organic Compounds with only single bonds. • “Saturated” • Methane is the simplest alkane.
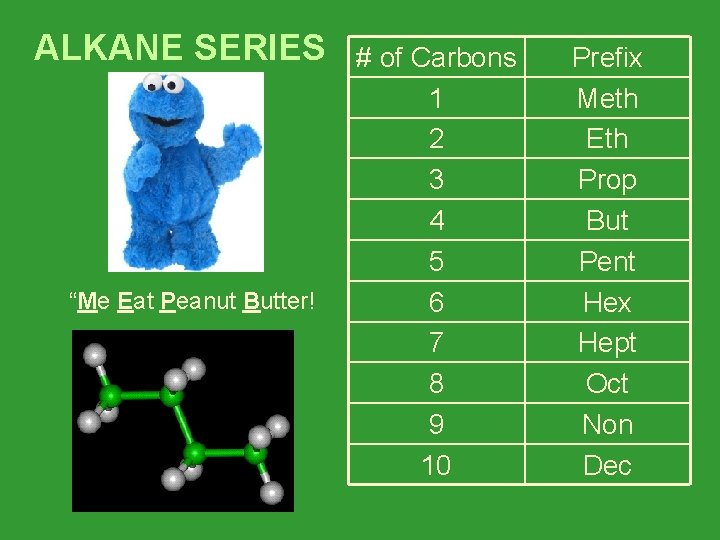
ALKANE SERIES “Me Eat Peanut Butter! # of Carbons 1 2 3 4 5 6 7 8 9 10 Prefix Meth Eth Prop But Pent Hex Hept Oct Non Dec
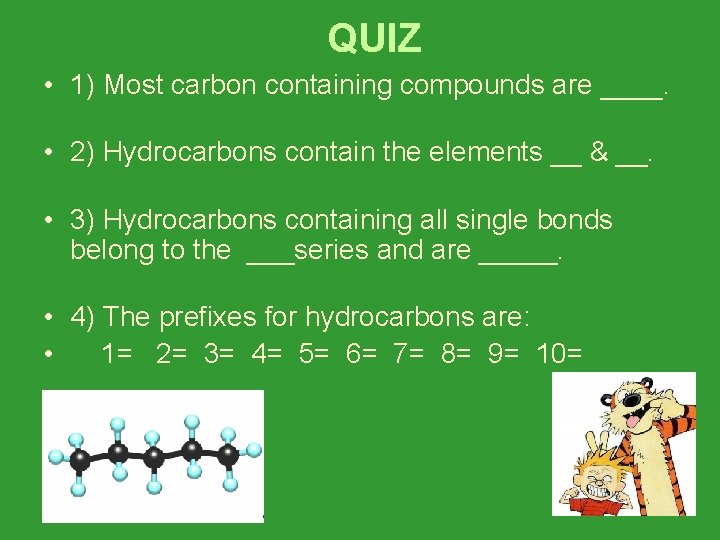
QUIZ • 1) Most carbon containing compounds are ____. • 2) Hydrocarbons contain the elements __ & __. • 3) Hydrocarbons containing all single bonds belong to the ___series and are _____. • 4) The prefixes for hydrocarbons are: • 1= 2= 3= 4= 5= 6= 7= 8= 9= 10=
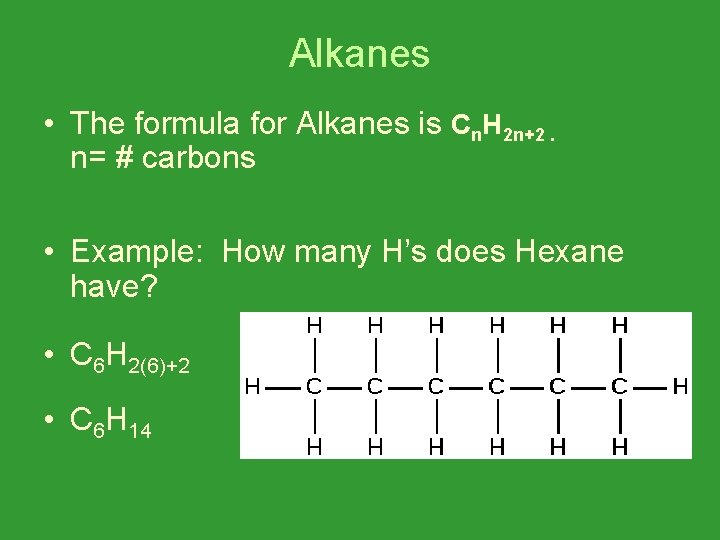
Alkanes • The formula for Alkanes is Cn. H 2 n+2. n= # carbons • Example: How many H’s does Hexane have? • C 6 H 2(6)+2 • C 6 H 14
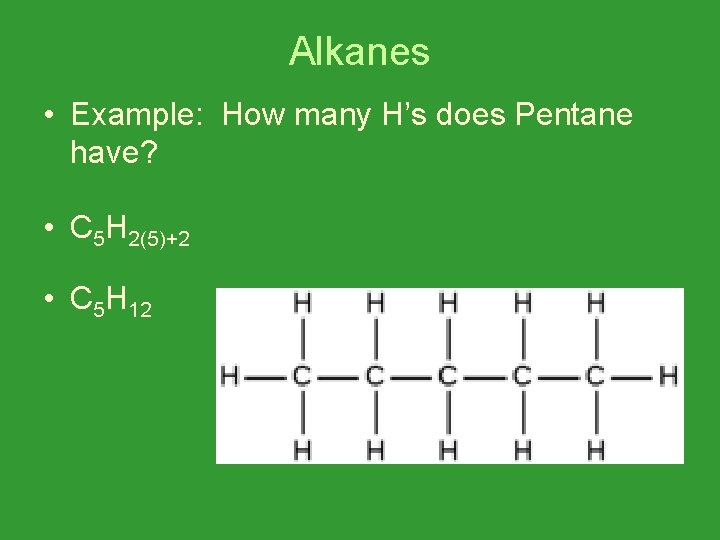
Alkanes • Example: How many H’s does Pentane have? • C 5 H 2(5)+2 • C 5 H 12
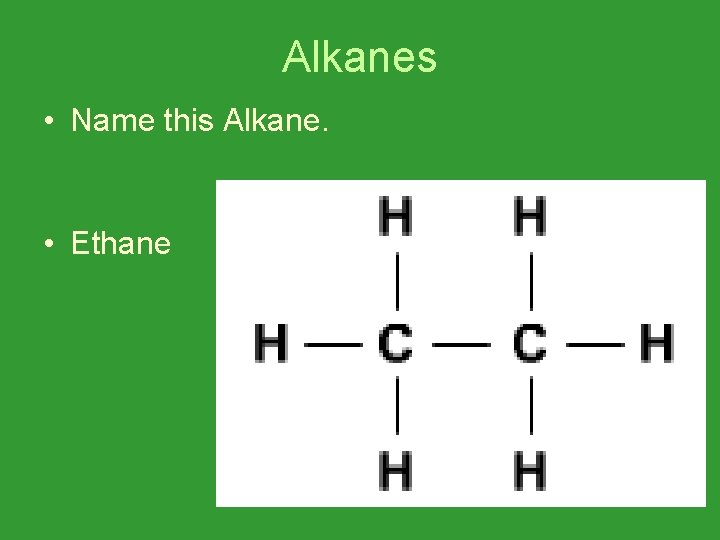
Alkanes • Name this Alkane. • Ethane
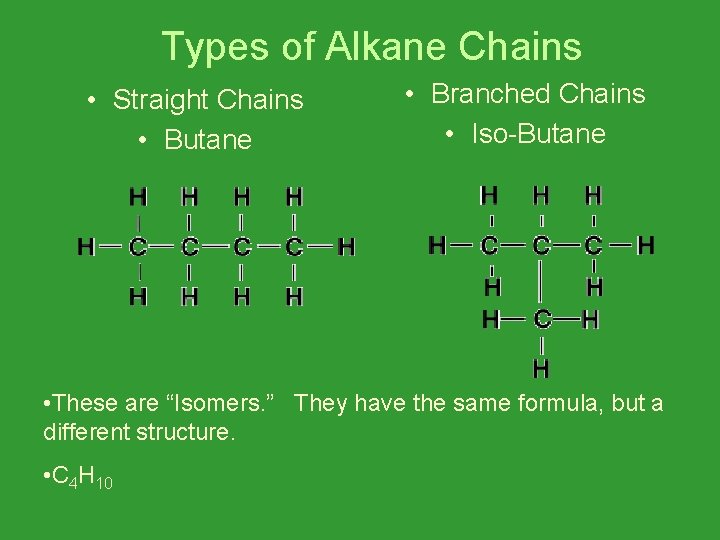
Types of Alkane Chains • Straight Chains • Butane • Branched Chains • Iso-Butane • These are “Isomers. ” They have the same formula, but a different structure. • C 4 H 10
Alkyl Groups • These are the branches of the chain. • “yl” endings
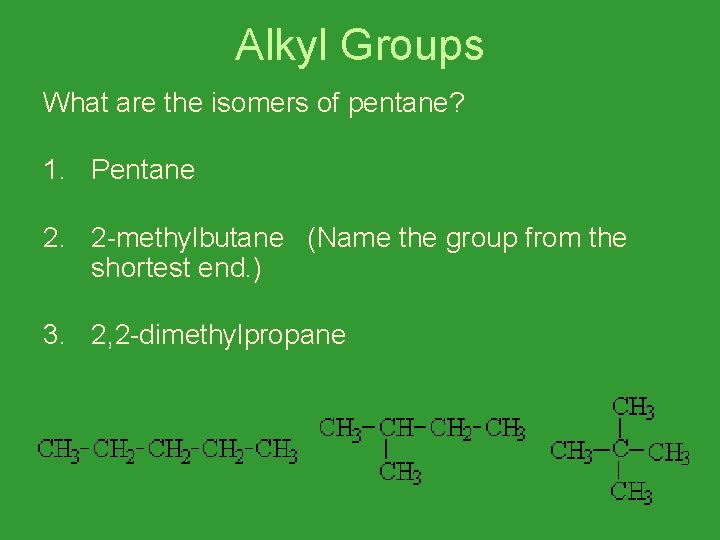
Alkyl Groups What are the isomers of pentane? 1. Pentane 2. 2 -methylbutane (Name the group from the shortest end. ) 3. 2, 2 -dimethylpropane

Naming Alkanes (IUPAC Rules) 1. Count # Carbons in longest continuous chain (parent chain). 2. Number Carbons in parent chain (choose a side so that substituent groups have the smallest sum). Name each alkyl group (“yl” ending). Place this name before name of parent chain. 3. If same alkyl group occurs more than once as a branch, use prefix (di-, tri-, tetra, etc. ) before the name to indicate the number of groups. 4. If different alkyl groups occur, name in alphabetical order. 5. Write entire name using hyphens (to separate numbers from words) and commas (to separate numbers).
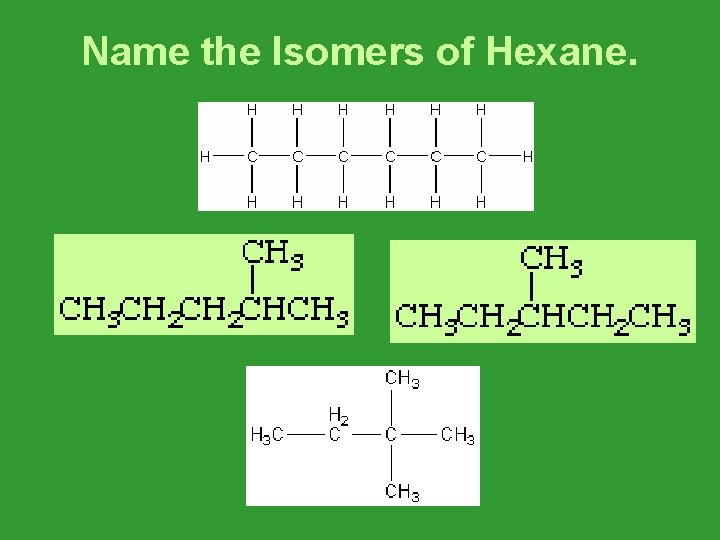
Name the Isomers of Hexane.
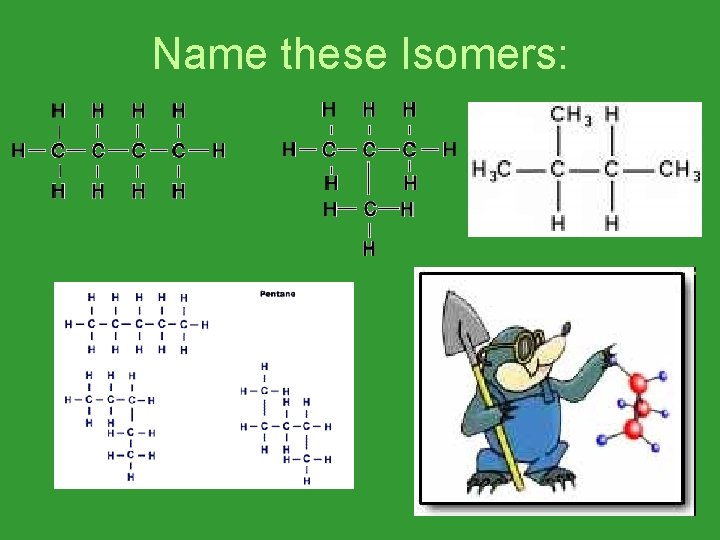
Name these Isomers:
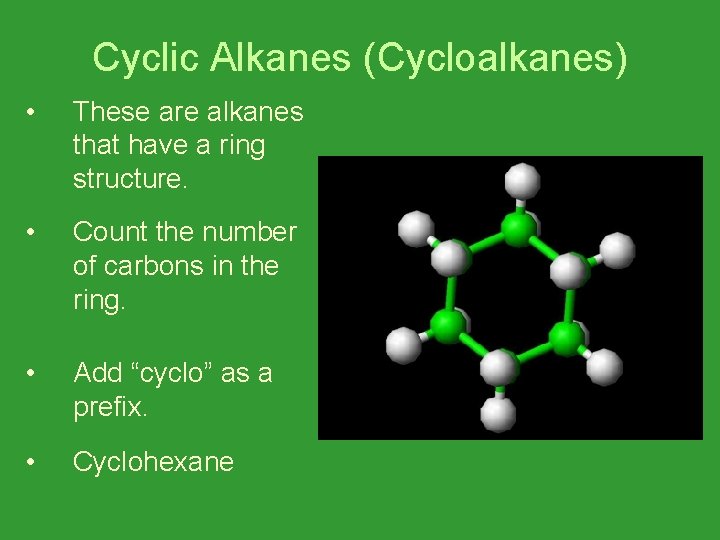
Cyclic Alkanes (Cycloalkanes) • These are alkanes that have a ring structure. • Count the number of carbons in the ring. • Add “cyclo” as a prefix. • Cyclohexane
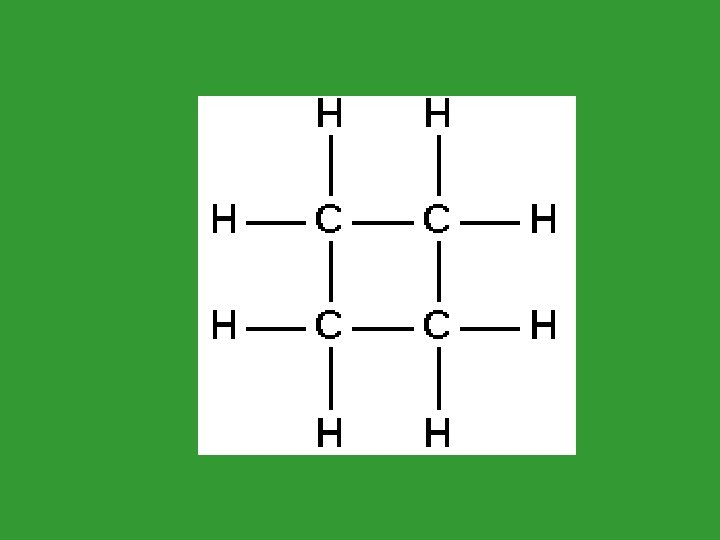
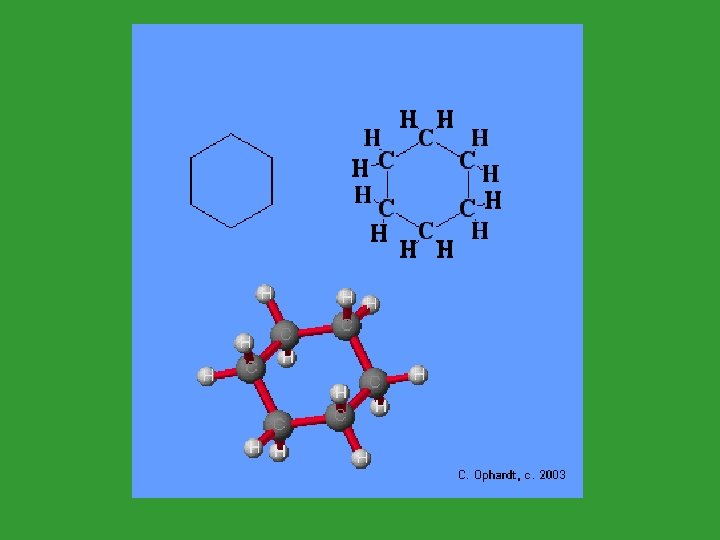

Alkane Quiz 1. Alkanes can be in straight chains or ____. 2. To show a branch, use the “___” ending. 3. Hydrocarbons that have the same formula but different structure are called ___. 4. ___ alkanes have a ring structure. They are named using the prefix “_____”.
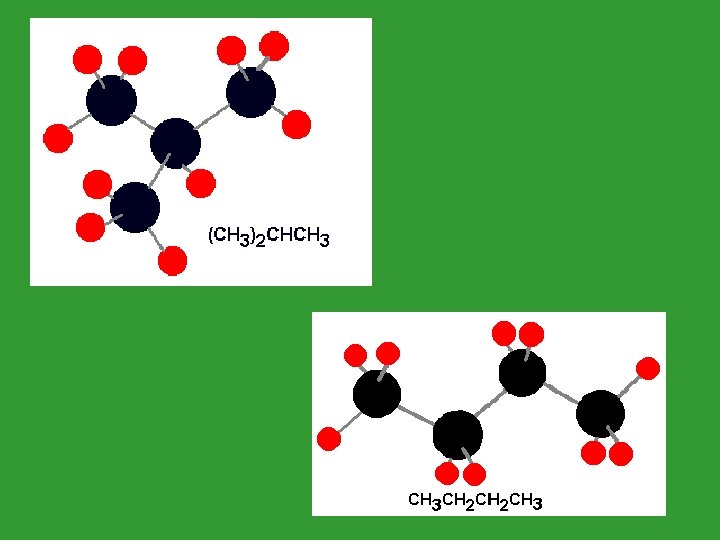

Properties of Alkanes • Alkanes are nonpolar covalent molecules • This means there is less attraction between molecules. • Therefore, alkanes are immiscible (insoluble) in water. (Water is polar).
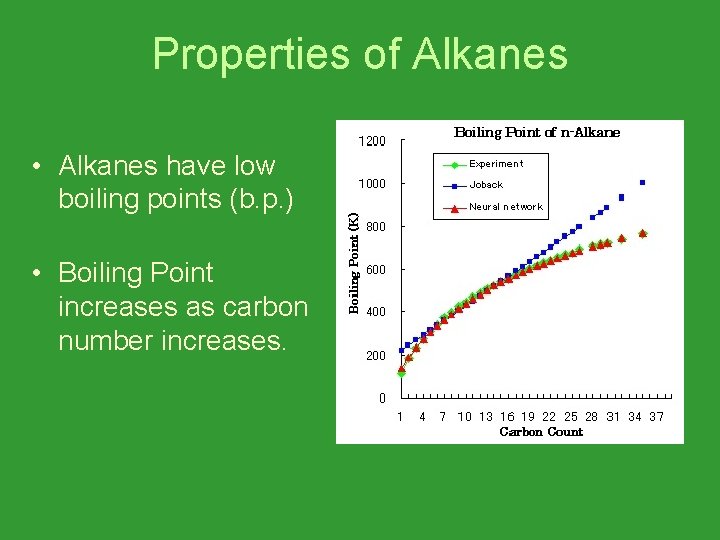
Properties of Alkanes • Alkanes have low boiling points (b. p. ) • Boiling Point increases as carbon number increases.

Properties of Alkanes • Due to nonpolar bonds and strong bonds between Carbon and Hydrogen, Alkanes have low reactivity. • Alkanes burn. CH 4 + 2 O 2 CO 2 + 2 H 2 O • Notice that combustion & respiration have similar products.

Properties of Alkanes Quiz 1. Alkanes are immiscible in water because they are ___. 2. They have ___ boiling points because they have __ intermolecular forces. 3. Boiling point __ as carbon number increases. 4. Alkanes show ___ reactivity because of strong H-C bonds. 5. Alkanes undergo ___ reactions (burning). 6. When alkanes burn, they react with __ in the air to form ____ and water. 7. Combustion has the same products as ____.
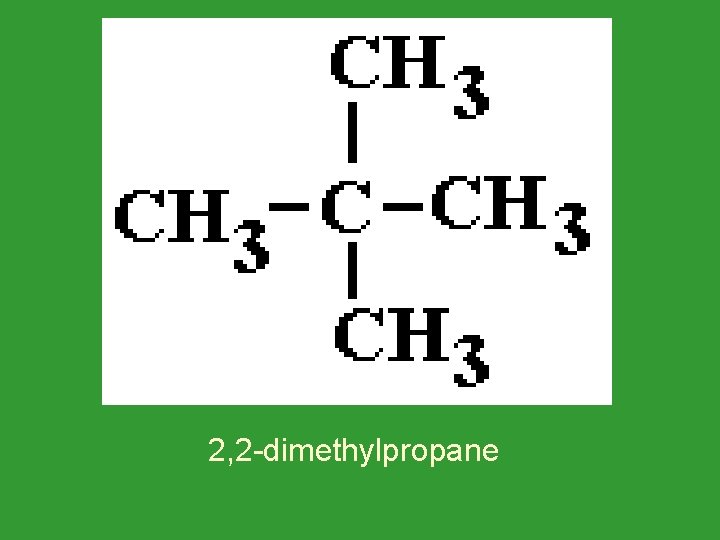
2, 2 -dimethylpropane
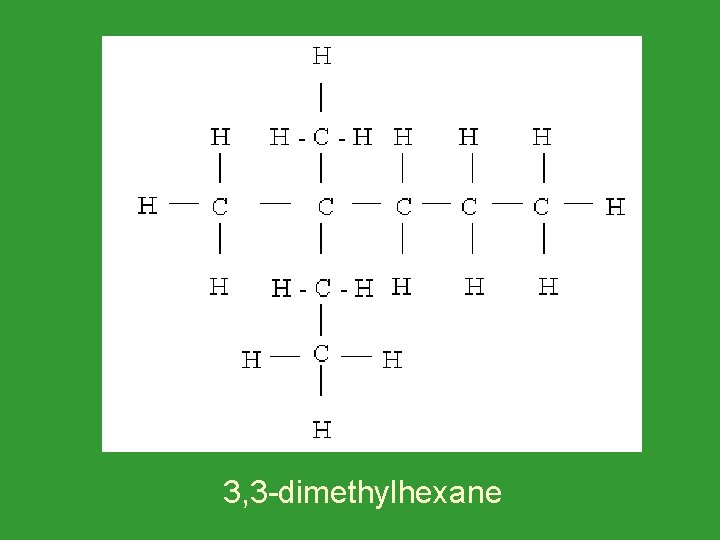
3, 3 -dimethylhexane
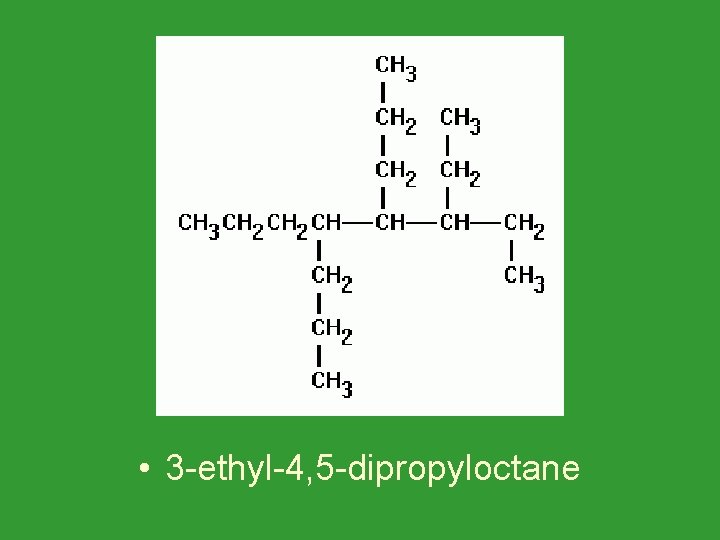
• 3 -ethyl-4, 5 -dipropyloctane
Alkene and Alkyne • Alkanes are organic compounds that only contain single bonds. • Alkenes are organic compounds that have double bonds. • Alkynes are organic compounds that have triple bonds.
Bonding The End
- Slides: 102