Bond Types Polarity Chemistry Joke Q What do
Bond Types / Polarity

Chemistry Joke Q: What do you pay a policeman who works security for a chemistry night class? A: Copper Nitrate!

Let’s Look At 3 Types of Bonds • Ionic • Polar Covalent (Molecular) • Nonpolar Covalent (Molecular)
Remember Electronegativity? • The tendency for an atom to attract electrons to itself in a bond – The higher the value, the better it is at attracting electrons. • The difference in the electronegativity values determines what type of bond will be formed.
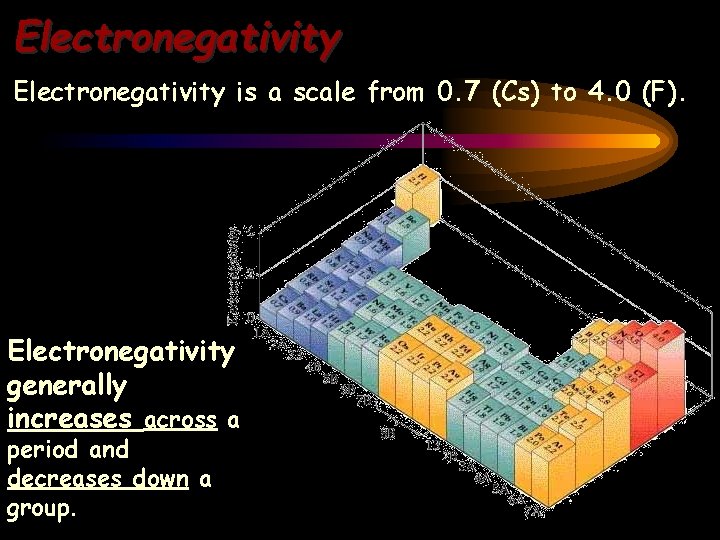
Electronegativity is a scale from 0. 7 (Cs) to 4. 0 (F). Electronegativity generally increases across a period and decreases down a group.

Electronegativity Values (on back of Periodic Table) • Why don’t the noble gases have a value? • They don’t attract electrons!
Ionic Bonds • If the electronegativity difference is great enough, one atom will pull the electron completely away from other atom—the electrons are NOT shared. • For example, electronegativity of Na is 0. 9; Cl is 3. 0. • This difference is very great, so the electrons are TRANSFERRED, forming ions • Positive and negative charges attract
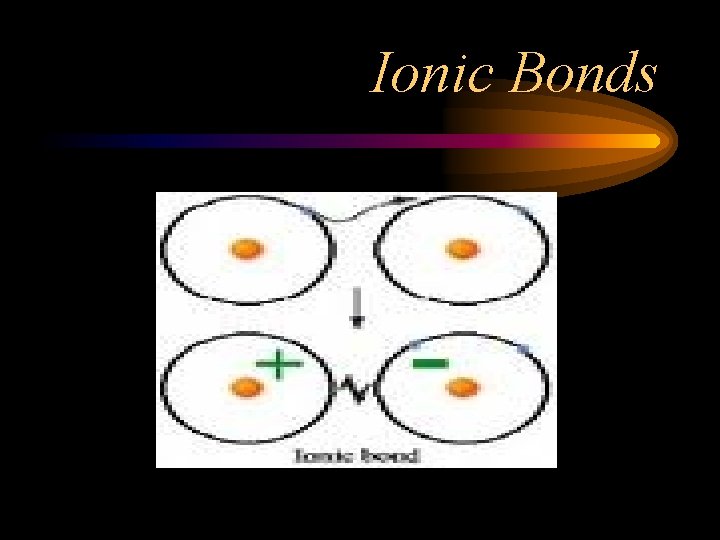
Ionic Bonds

Polar Covalent Bonds • Covalent bonds share electrons • The shared pairs are pulled, similar to a tug-of-war, between the nuclei of the atoms sharing the electrons. • If the electronegativity difference is large enough, one side of the bond becomes slightly more negative and the other side becomes slightly more positive. • This is a Polar Covalent Bond
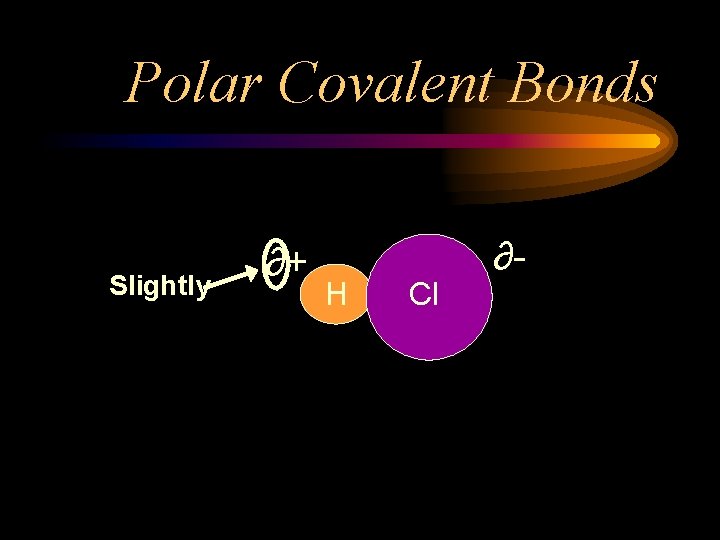
Polar Covalent Bonds Slightly ∂+ H Cl ∂-
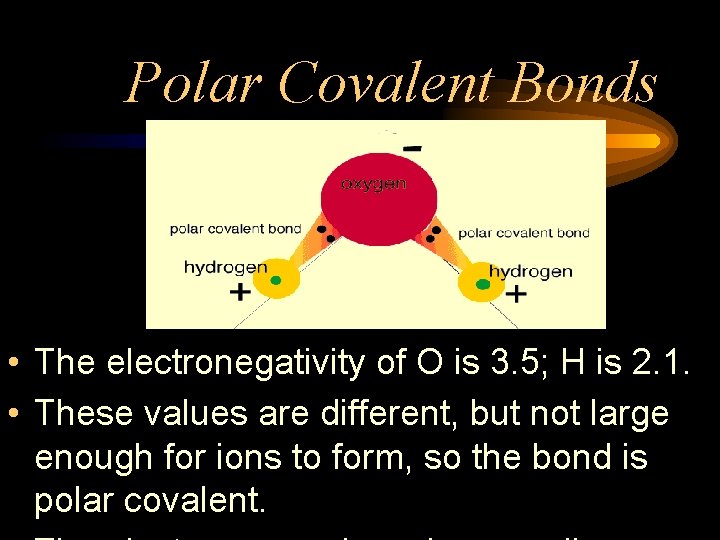
Polar Covalent Bonds • The electronegativity of O is 3. 5; H is 2. 1. • These values are different, but not large enough for ions to form, so the bond is polar covalent.
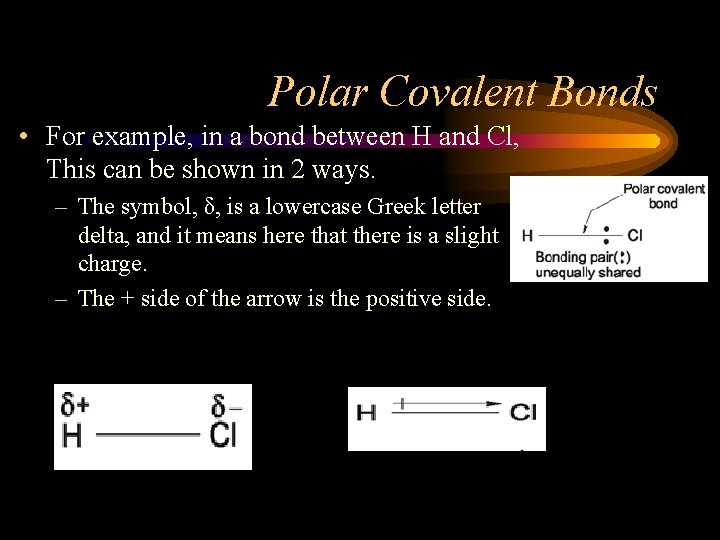
Polar Covalent Bonds • For example, in a bond between H and Cl, This can be shown in 2 ways. – The symbol, δ, is a lowercase Greek letter delta, and it means here that there is a slight charge. – The + side of the arrow is the positive side.
Nonpolar Covalent Bonds • When the atoms have similar (or identical) electronegativity), the electrons are equally shared, and the bond is Nonpolar Covalent • Neither side of the bond is even slightly positive or negative. • This is the type of bond that occurs between 2 atoms of the same element.
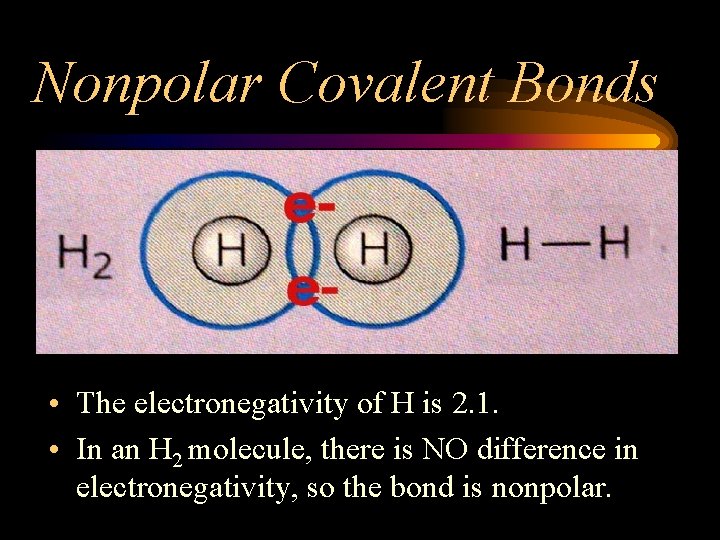
Nonpolar Covalent Bonds • The electronegativity of H is 2. 1. • In an H 2 molecule, there is NO difference in electronegativity, so the bond is nonpolar.
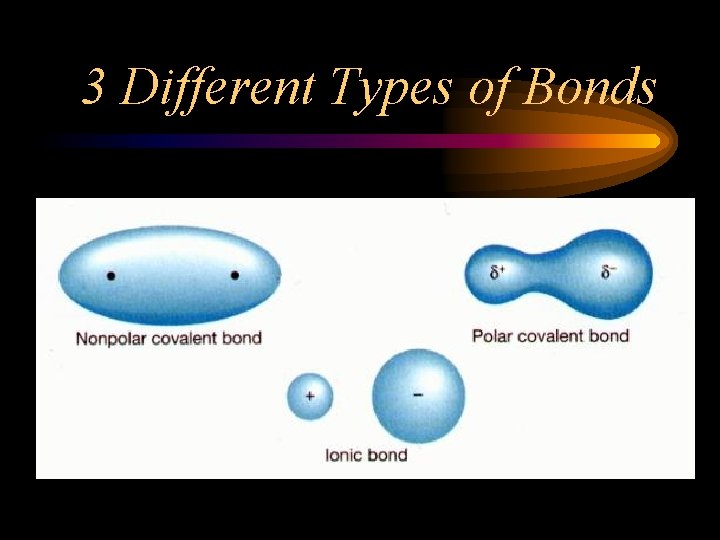
3 Different Types of Bonds

Nonpolar or Polar Molecules • We now know how to determine if the bond b/w atom and atom in a compound is polar or nonpolar. • But…what about the whole molecule? • Sometimes polar bonds are

Nonpolar or Polar Molecules How can you tell? • Draw the Lewis Structure. • If the central atom has any unshared pairs, the molecule is polar.

Nonpolar or Polar Molecules • If there are no unshared pairs on the central atom, look at the atoms around the central atom. – If they are all the same, the molecule is nonpolar. – If any one of them is different, the molecule is polar. • In a 2 -atom molecule, if the bond between

Nonpolar or Polar Molecules • Lone pairs of electrons on O H 2 O Polar • No lone pairs on C; two of the CO 2 Nonpola same type of atoms bonded to C r • H and Cl are two different atoms Pol HC bonded together ar l. HCN Pol • No lone pairs on C; two different ar types of atoms bonded to C N 2 Nonpola • Two identical atoms bonded r together CH 3 Cl Pol • One of the atoms bonded to C is ar different

Chemistry Joke Q: How do we know that Chuck Norris is not a chemist? 110 A: The only Su element he knows (268) is the “element” of
- Slides: 21