Bond Enthalpy Chem 12 The enthalpy change required

Bond Enthalpy Chem 12
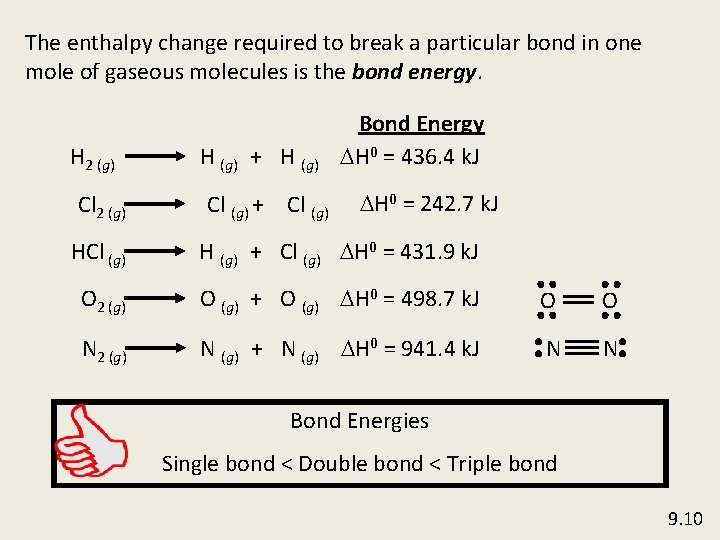
The enthalpy change required to break a particular bond in one mole of gaseous molecules is the bond energy. H 2 (g) Cl 2 (g) H (g) + H (g) Cl (g) + Cl (g) Bond Energy DH 0 = 436. 4 k. J DH 0 = 242. 7 k. J HCl (g) H (g) + Cl (g) DH 0 = 431. 9 k. J O 2 (g) O (g) + O (g) DH 0 = 498. 7 k. J O O N 2 (g) N (g) + N (g) DH 0 = 941. 4 k. J N N Bond Energies Single bond < Double bond < Triple bond 9. 10
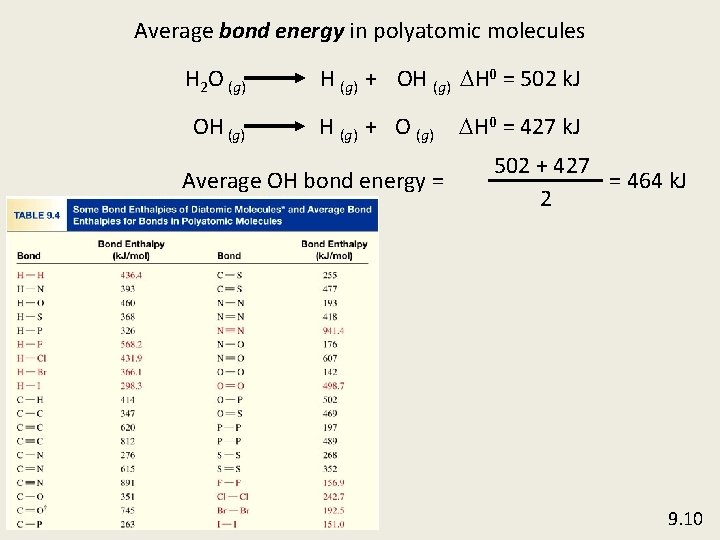
Average bond energy in polyatomic molecules H 2 O (g) OH (g) + OH (g) DH 0 = 502 k. J H (g) + O (g) Average OH bond energy = DH 0 = 427 k. J 502 + 427 = 464 k. J 2 9. 10
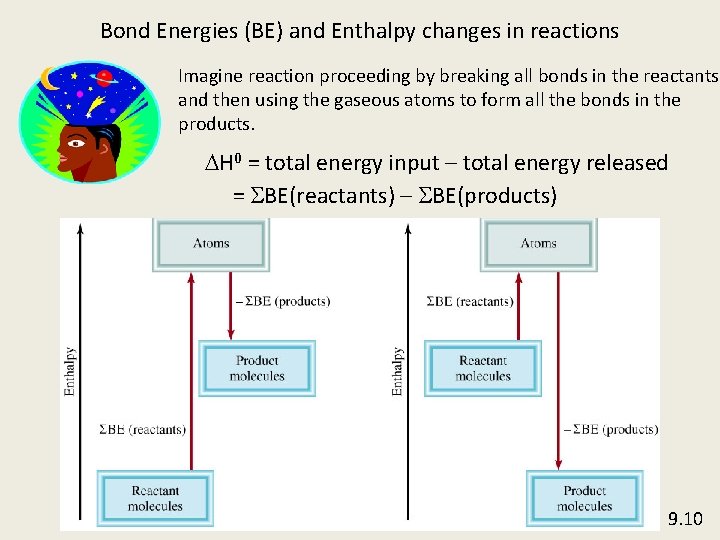
Bond Energies (BE) and Enthalpy changes in reactions Imagine reaction proceeding by breaking all bonds in the reactants and then using the gaseous atoms to form all the bonds in the products. DH 0 = total energy input – total energy released = SBE(reactants) – SBE(products) 9. 10
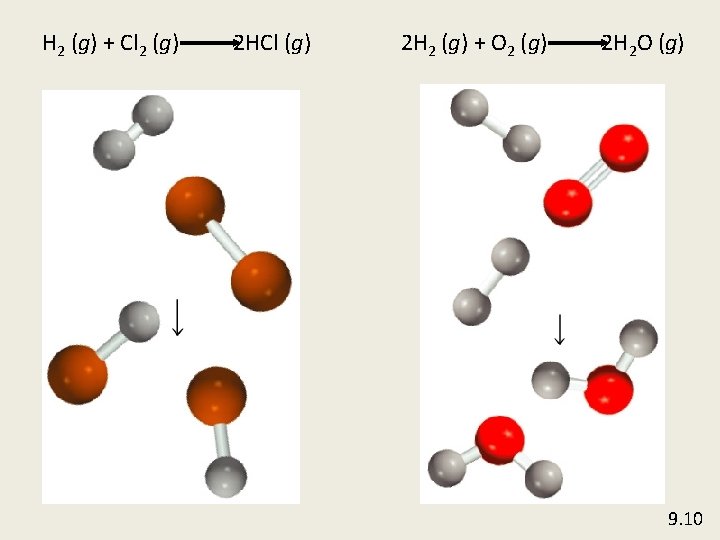
H 2 (g) + Cl 2 (g) 2 HCl (g) 2 H 2 (g) + O 2 (g) 2 H 2 O (g) 9. 10
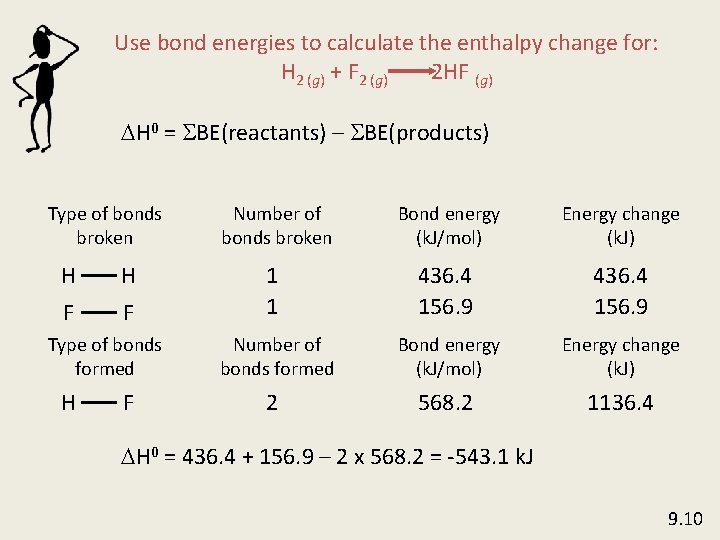
Use bond energies to calculate the enthalpy change for: H 2 (g) + F 2 (g) 2 HF (g) DH 0 = SBE(reactants) – SBE(products) Type of bonds broken H H F F Type of bonds formed H F Number of bonds broken Bond energy (k. J/mol) Energy change (k. J) 1 1 436. 4 156. 9 Number of bonds formed Bond energy (k. J/mol) Energy change (k. J) 2 568. 2 1136. 4 DH 0 = 436. 4 + 156. 9 – 2 x 568. 2 = -543. 1 k. J 9. 10
- Slides: 6