Bohrs Model The Quantum Mechanical Model What we
Bohr’s Model The Quantum Mechanical Model

What we know about the atom up ‘til now. . .

• Rutherford's model could not explain why metals or compounds of metals give off characteristic colors when heated with a flame.
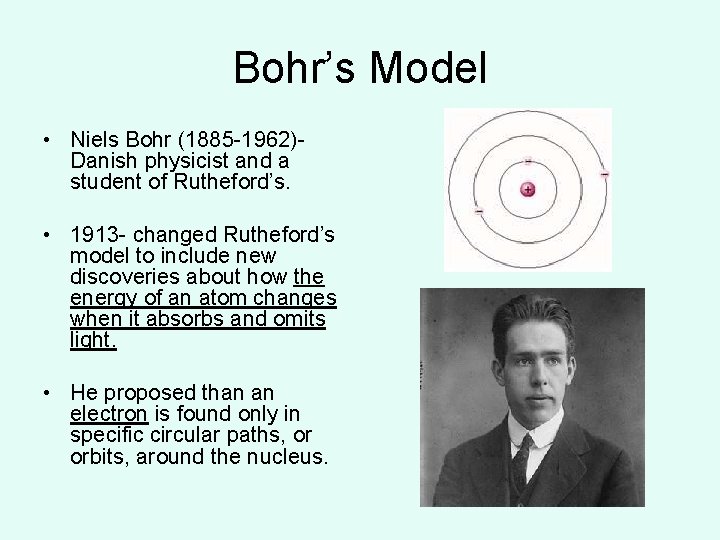
Bohr’s Model • Niels Bohr (1885 -1962)Danish physicist and a student of Rutheford’s. • 1913 - changed Rutheford’s model to include new discoveries about how the energy of an atom changes when it absorbs and omits light. • He proposed than an electron is found only in specific circular paths, or orbits, around the nucleus.
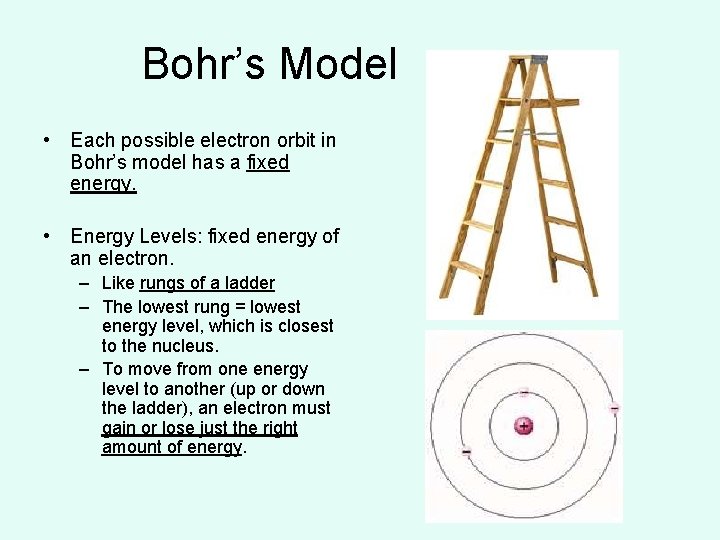
Bohr’s Model • Each possible electron orbit in Bohr’s model has a fixed energy. • Energy Levels: fixed energy of an electron. – Like rungs of a ladder – The lowest rung = lowest energy level, which is closest to the nucleus. – To move from one energy level to another (up or down the ladder), an electron must gain or lose just the right amount of energy.
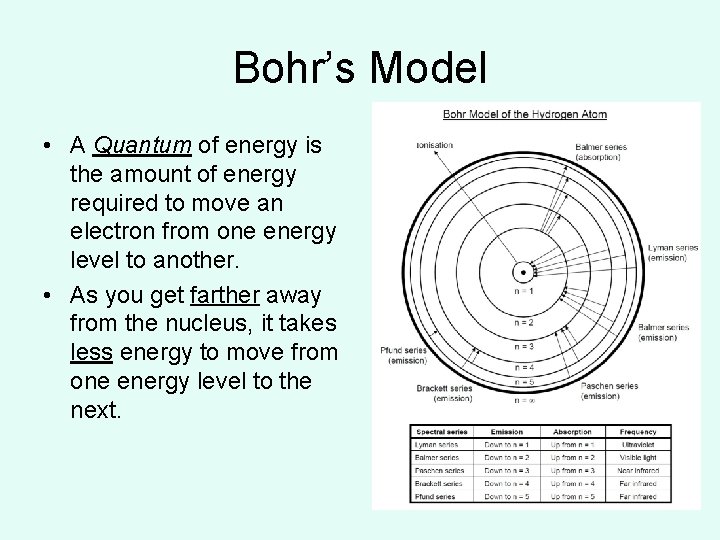
Bohr’s Model • A Quantum of energy is the amount of energy required to move an electron from one energy level to another. • As you get farther away from the nucleus, it takes less energy to move from one energy level to the next.
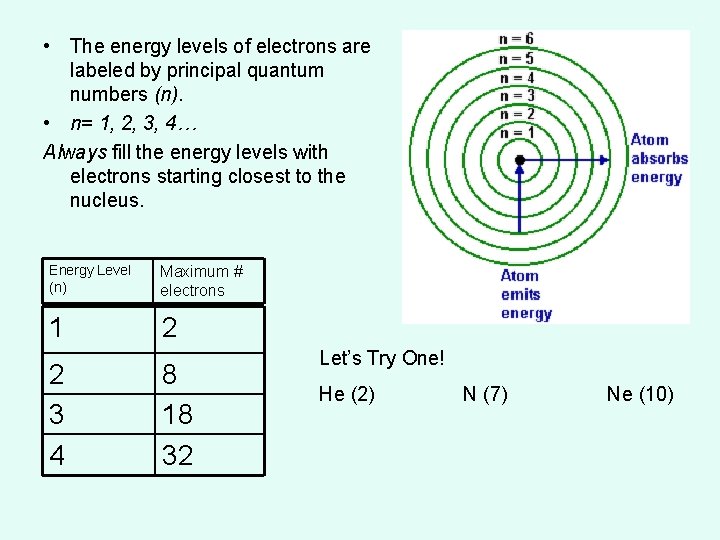
• The energy levels of electrons are labeled by principal quantum numbers (n). • n= 1, 2, 3, 4… Always fill the energy levels with electrons starting closest to the nucleus. Energy Level (n) Maximum # electrons 1 2 2 3 4 8 18 32 Let’s Try One! He (2) N (7) Ne (10)
Electron Placement Activity! • • Periodic Table Packet Pen/pencil Partner
Where Bohr was wrong… • This model could explain Hydrogen (1 electron) but failed to explain the energies absorbed and emitted by atoms with more than one electron.
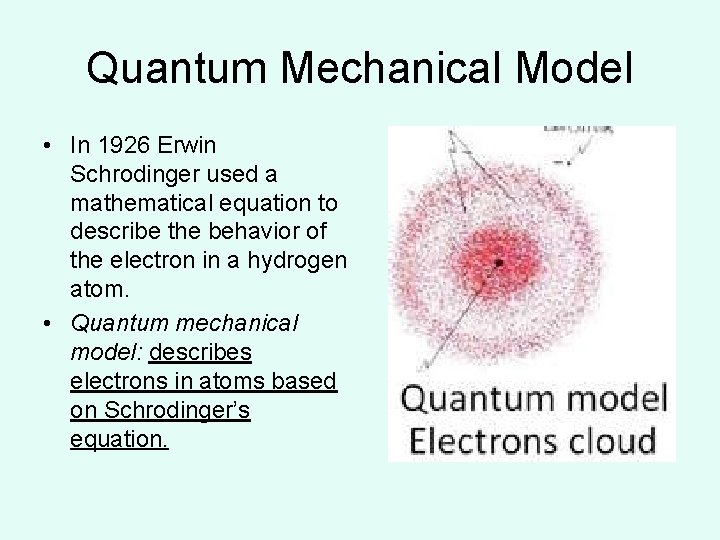
Quantum Mechanical Model • In 1926 Erwin Schrodinger used a mathematical equation to describe the behavior of the electron in a hydrogen atom. • Quantum mechanical model: describes electrons in atoms based on Schrodinger’s equation.
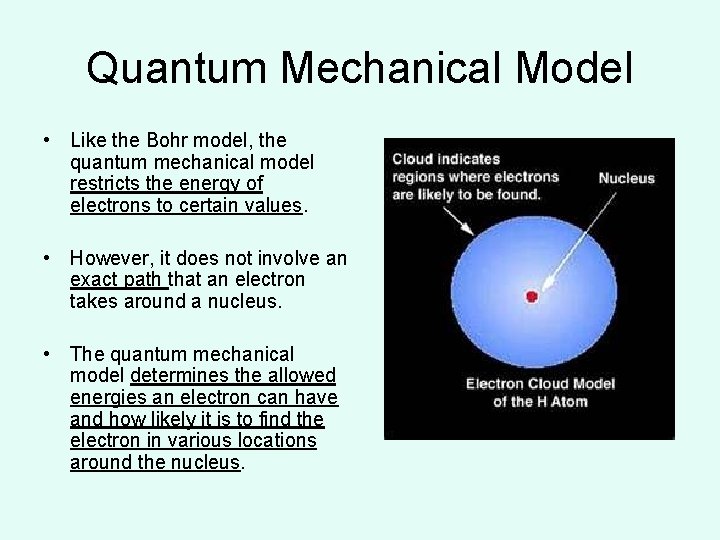
Quantum Mechanical Model • Like the Bohr model, the quantum mechanical model restricts the energy of electrons to certain values. • However, it does not involve an exact path that an electron takes around a nucleus. • The quantum mechanical model determines the allowed energies an electron can have and how likely it is to find the electron in various locations around the nucleus.
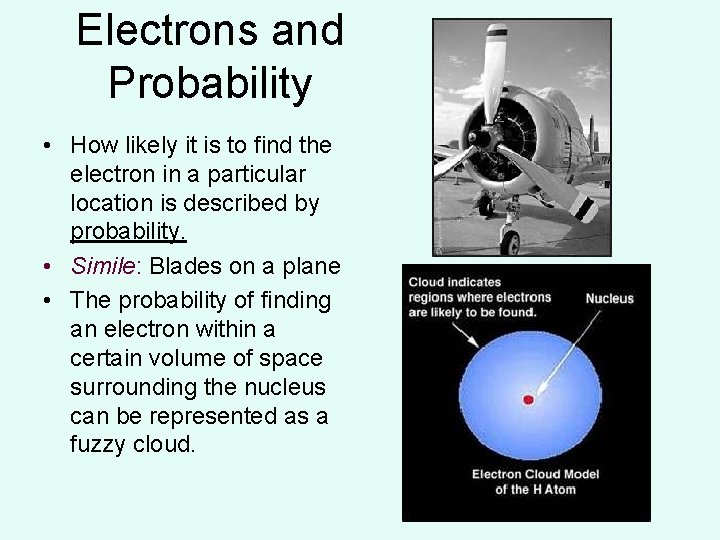
Electrons and Probability • How likely it is to find the electron in a particular location is described by probability. • Simile: Blades on a plane • The probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud.
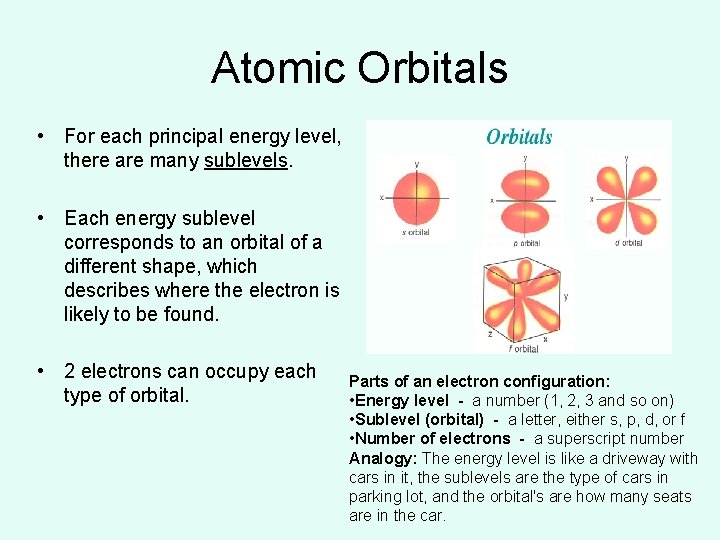
Atomic Orbitals • For each principal energy level, there are many sublevels. • Each energy sublevel corresponds to an orbital of a different shape, which describes where the electron is likely to be found. • 2 electrons can occupy each type of orbital. Parts of an electron configuration: • Energy level - a number (1, 2, 3 and so on) • Sublevel (orbital) - a letter, either s, p, d, or f • Number of electrons - a superscript number Analogy: The energy level is like a driveway with cars in it, the sublevels are the type of cars in parking lot, and the orbital's are how many seats are in the car.
• Quantum Mechanics • http: //www. teachersd omain. org/asset/phy 0 3_vid_quantum/ • http: //www. teachersd omain. org/asset/phy 0 3_vid_atoms/
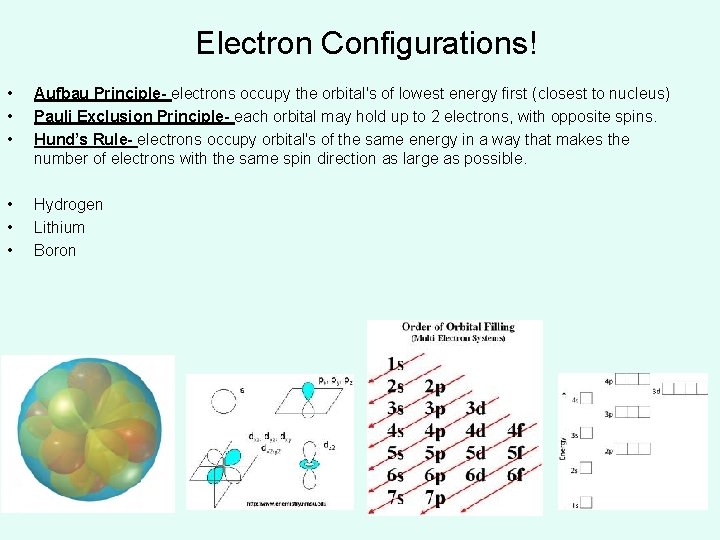
Electron Configurations! • • • Aufbau Principle- electrons occupy the orbital's of lowest energy first (closest to nucleus) Pauli Exclusion Principle- each orbital may hold up to 2 electrons, with opposite spins. Hund’s Rule- electrons occupy orbital's of the same energy in a way that makes the number of electrons with the same spin direction as large as possible. • • • Hydrogen Lithium Boron
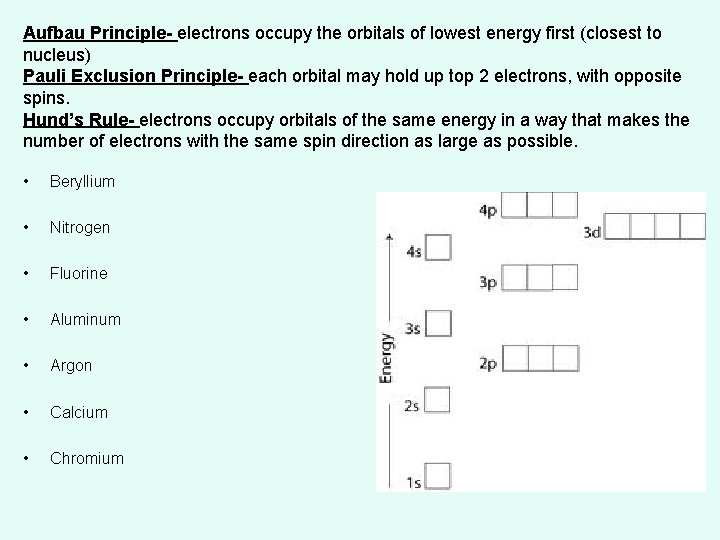
Aufbau Principle- electrons occupy the orbitals of lowest energy first (closest to nucleus) Pauli Exclusion Principle- each orbital may hold up top 2 electrons, with opposite spins. Hund’s Rule- electrons occupy orbitals of the same energy in a way that makes the number of electrons with the same spin direction as large as possible. • Beryllium • Nitrogen • Fluorine • Aluminum • Argon • Calcium • Chromium
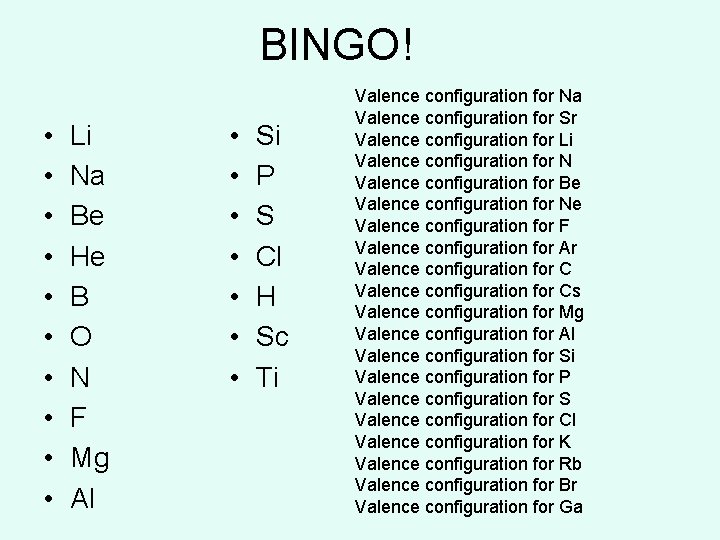
BINGO! • • • Li Na Be He B O N F Mg Al • • Si P S Cl H Sc Ti Valence configuration for Na Valence configuration for Sr Valence configuration for Li Valence configuration for N Valence configuration for Be Valence configuration for Ne Valence configuration for F Valence configuration for Ar Valence configuration for Cs Valence configuration for Mg Valence configuration for Al Valence configuration for Si Valence configuration for P Valence configuration for S Valence configuration for Cl Valence configuration for K Valence configuration for Rb Valence configuration for Br Valence configuration for Ga
In Bohr’s model of the atom, where are the electrons and protons? a. The electrons move around the protons, which are at the center of the atom b. The electrons and protons move throughout the atom c. The electrons occupy fixed orbits around the protons, which are at the center of the atom. d. The electrons and protons are located throughout the atom, but they are not free to move.
Which is NOT true for the Bohr model? • A. electrons can jump to higher energy levels if they receive the correct amount of energy • B. The electrons circle the nucleus in orbits • C. Electrons may occupy regions of space between the circular orbits • D. When electrons jump to a lower energy level, they release energy in the form of light.

• The principal quantum number indicated what property of an electron? a. Position b. Speed c. Energy level d. Electron cloud shape

What letter identifies the principle quantum number (aka energy level)? • • A. c B. n C. q D. f

• The smallest particle of an element that retains the properties of that element is a(n) • A. atom • B. electron • C. proton • D. neutron
• • • The subatomic particles of an atom are A. Protons and neutrons B. Protons and electrons C. Electrons and Neutrons D. Protons, neutrons, and electrons
• • What two particles are in the nucleus? A. protons and electrons B. neutrons and electrons C. protons and neutrons

• • What is the charge of an electron? A. Negative B. Positive C. Neutral

• What was Rutheford’s model of the atom? • A. A dense sphere with no charge • B. A tiny sphere with negative charged particles throughout • C. A dense nucleus center with orbiting electrons

• Dalton thought the atom was a • A. tiny, dense sphere with no charge • B. A tiny sphere with negatively charged particles throughout • C. A positively centered nucleus with negative electrons orbiting around it

• J. J. Thompson discovered the electron with the use of the • A. Gold Foil experiment • B. Cathode Ray Tube • C. an electron microscope

All atoms are • A. positively charged, the number of protons exceeding the number of electrons • B. negatively charged, with the number of electrons exceeding the number of protons • C. neutral, with the number of protons equaling the number of electrons • D. neutral, with the number of protons equaling he number of electrons, which is equal to the number of neutrons.

A theory is • A. proposed explanation for an observation • B. well tested explanation for a broad set of observations • C. summary of the results of many observations • D. procedure used to test a proposed explanation.

Which of the following is NOT a physical property • • A. mass B. color C. Ability to rust D. melting point

When a substance forms a vapor, what physical state was it before? • • A. gas B. liquid C. solid D. liquid or solid

What state of matter has no definite shape or volume? • • A. solid B. Liquid C. Gas D. Both a and c

Which state has a definite volume but no definite shape? • • A. gas B. Solid C. Liquid D. both a and c

Which state of matter has both a definite shape and definite volume? • • A. solid B. Liquid C. Gas D. both a and c

What is another word for a homogeneous solution? • • A. Solutions B. Homozygous mixture C. Mixture D. Compound

• • Which of the following is an example of a homogeneous mixture? A. Oil and water B. Rubbing alcohol C. Italian salad dressing D. Raw milk

Which is true for compounds? • A. They can be physically separated • B. They have compounds that vary • C. They are substances (aka pure substances) • D. They have similar properties to elements.

Which of the following is a chemical property? • • A. hardness B. Color C. melting point D. ability to react with oxygen

When metal rusts, it is evidence of a • • A. Physical change B. Chemical change C. homogeneous solution D. heterogeneous solution

What is the difference between a mixture and a substance (aka pure substance) • A. substance are compounds, mixtures are not • B. Mixtures are groups of elements and compounds arent • C. Sample of the same substance have different properties • D. Mixtures can be physically separated, while compounds can’t.

Quick Review • In scientific method: • Another word for independent variable is manipulated variable. • Another word for dependent variable is responding variable

The variable that is observed during an experiment is called what type of variable? • • A. independent B. manipulated C. control D. responding

All of these are examples are physical changes EXCEPT: • • A. bending B. cutting C. rusting D. polishing

During a chemical reaction • • A. matter is neither created nor destroyed B. some matter is destroyed C. some matter is created D. some matter is destroyed, some is created

The number 2012 in scientific notation is • • A. 2. 012 x 10^3 B. 2. 012 x 10^-3 C. 20. 12 x 10^-3 D. 2. 012 x 10^4

In the measurement 0. 709 ml, which digit is the least precise? • • A. 7 B. 0 C. 9 D. all numbers

In the measurement, . 6745 g, which number is the estimated digit? • • A. 6 B. 4 C. 5 D. 7

When a measurement is close to the correct value, it has good • • A. Precision B. Accuracy C. Usefulness D. Reproducibility

• • The closeness of your measurement to those of your lab partners indicates A. accuracy B. precision C. reproducibility D. usefulness

How many significant figures are in 3008? • • A. 2 B. 4 C. 1 D. 5
How many sig figs are in 20. 0090? • • A. 2 B. 6 C. 5 D. 3

Round 1050 to two significant figures • A. 1. 0 x 10^3
- Slides: 53