Bohrs Model of the Atom Spectral Lines Photons
Bohr’s Model of the Atom Spectral Lines, Photons, and Quantum Mechanics!
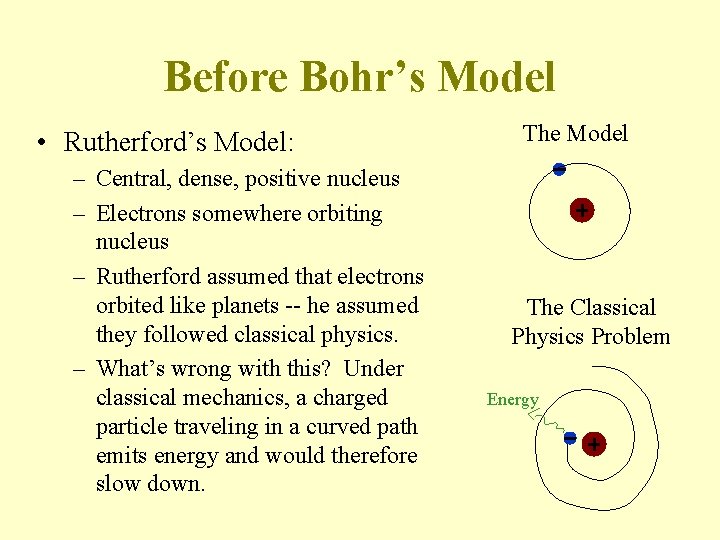
Before Bohr’s Model • Rutherford’s Model: – Central, dense, positive nucleus – Electrons somewhere orbiting nucleus – Rutherford assumed that electrons orbited like planets -- he assumed they followed classical physics. – What’s wrong with this? Under classical mechanics, a charged particle traveling in a curved path emits energy and would therefore slow down. The Model The Classical Physics Problem Energy
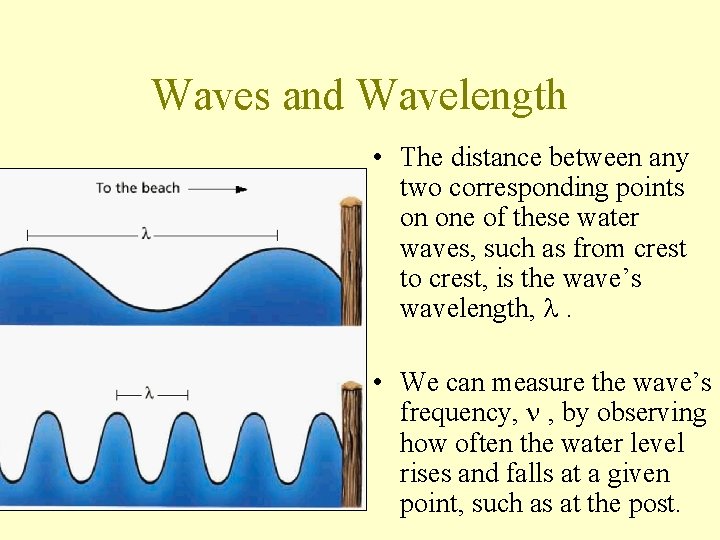
Waves and Wavelength • The distance between any two corresponding points on one of these water waves, such as from crest to crest, is the wave’s wavelength, l. • We can measure the wave’s frequency, n , by observing how often the water level rises and falls at a given point, such as at the post.

Background on Light • Wave nature of light: – Light travels in a similar way to waves in water or Slinky – c = ln • c = speed of light = 3. 0 x 108 m/s • l = wavelength • n = frequency l • Particle nature of light – Max Planck proposed that objects emit energy in small, specific amounts called quanta. – Einstein expanded on this, proposing that light is made of particles, called photons. These carry a quantum of energy, different for each wavelength.
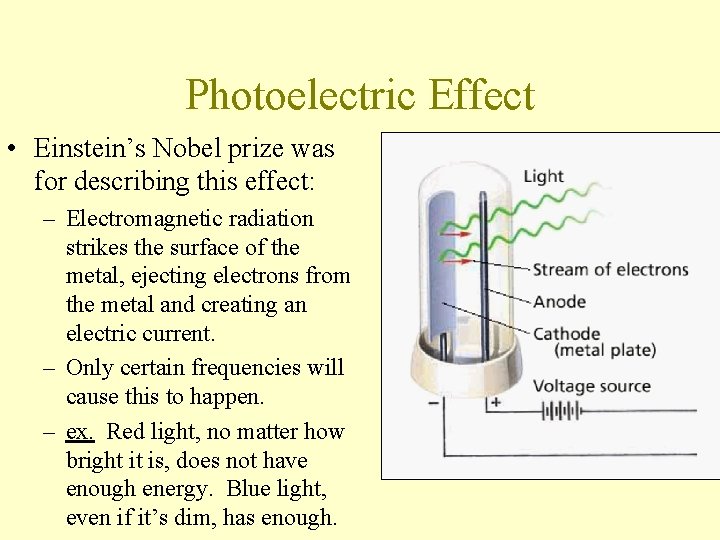
Photoelectric Effect • Einstein’s Nobel prize was for describing this effect: – Electromagnetic radiation strikes the surface of the metal, ejecting electrons from the metal and creating an electric current. – Only certain frequencies will cause this to happen. – ex. Red light, no matter how bright it is, does not have enough energy. Blue light, even if it’s dim, has enough.

Bohr’s Idea • Bohr said electrons were in set orbits, like rungs of a ladder. – You can only be on one orbit (rung) or the next, not in the middle -- they are quantized • Electrons can only move from one to the other by emitting or absorbing a particular wavelength of light (a photon with a certain energy), similar to the metal in the photoelectric effect.
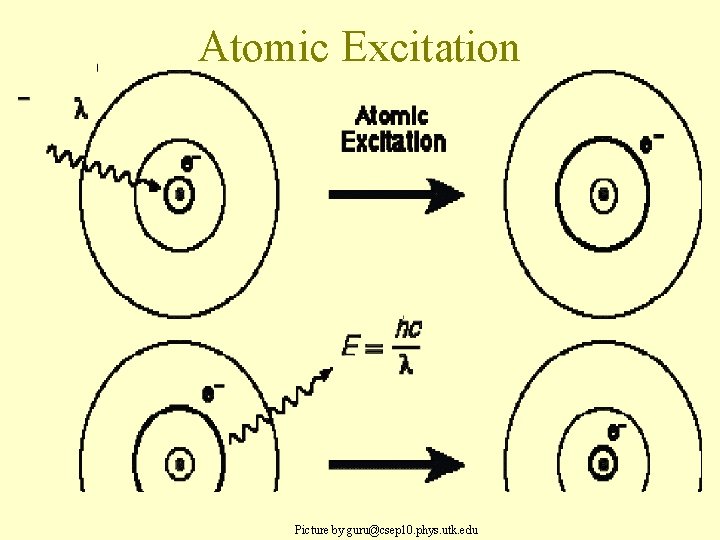
Atomic Excitation Picture by guru@csep 10. phys. utk. edu

Is there proof? • Normal Spectrum of Light: – White light, split up with a prism, looks like a continuous spectrum: • Spectral lines: – When an element is excited, there are lines rather than a spectrum of light. – To go from an excited state to the ground state, atoms must emit a certain frequency of light. These are constant for each element, as the electrons return from various outer “orbits. ”

How is this used? • Astronomers use these unique patterns to identify the composition and heat of stars. • This is how we know that stars are 90% hydrogen.

Seeing these lines on Earth • Spectral lines can be observed by sending electric current through a type of light bulb which is filled with the gas form of a given element and using a prism to break up the light emitted. – ex. hydrogen, neon, mercury • Absorption lines can be observed when a colored, transparent substance is held in front of a white line.
- Slides: 10