Bohrs Model of the Atom Bohrs Model Electron
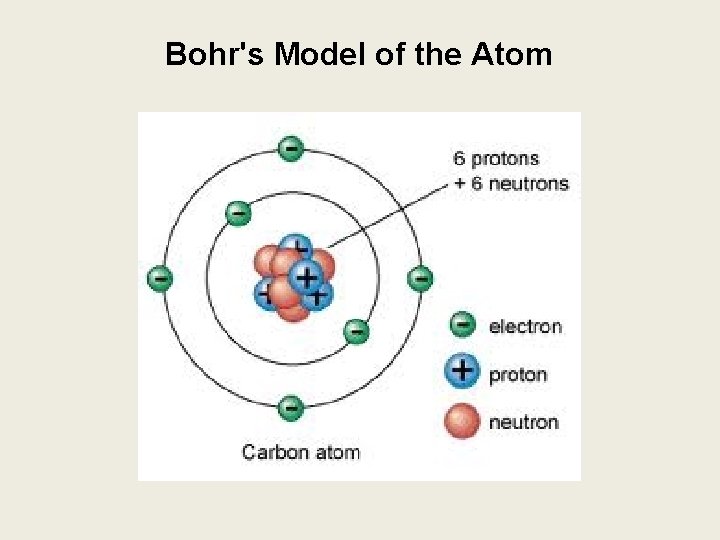
Bohr's Model of the Atom
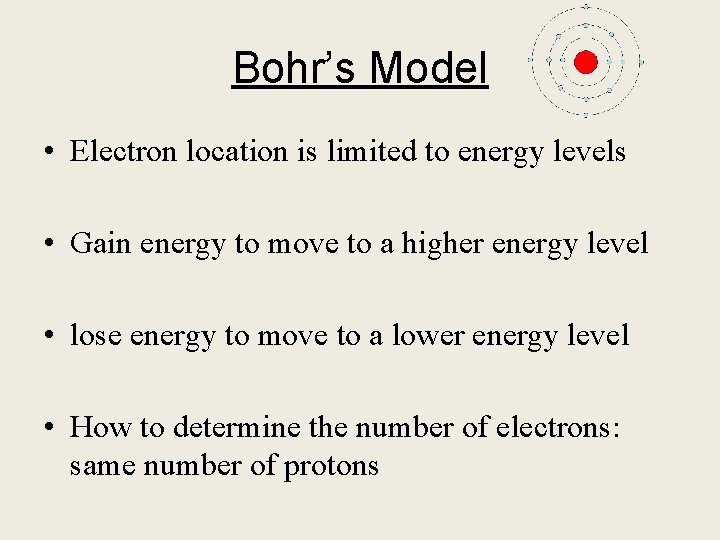
Bohr’s Model • Electron location is limited to energy levels • Gain energy to move to a higher energy level • lose energy to move to a lower energy level • How to determine the number of electrons: same number of protons
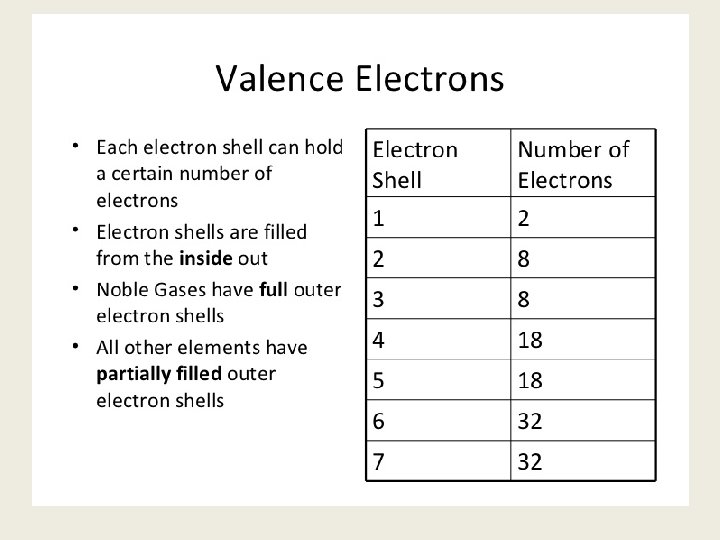
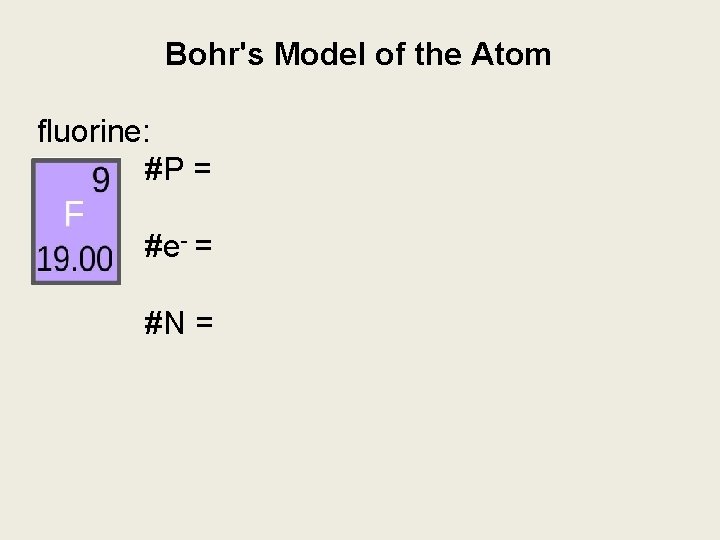
Bohr's Model of the Atom fluorine: #P = #e- = #N =
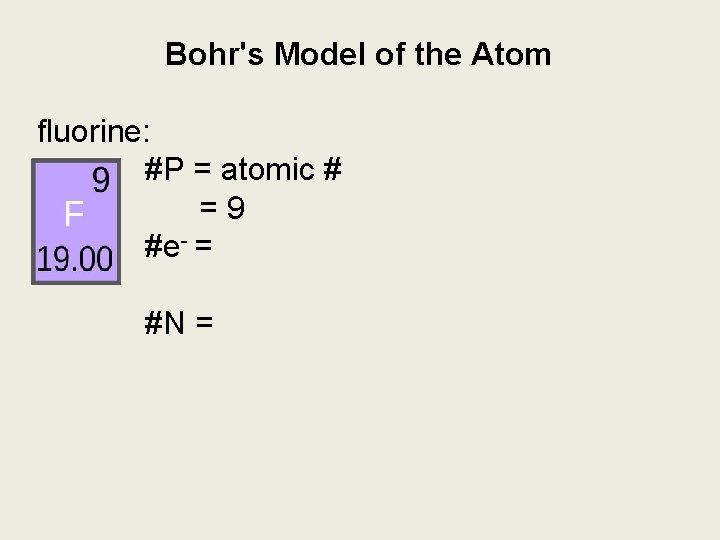
Bohr's Model of the Atom fluorine: #P = atomic # =9 #e- = #N =
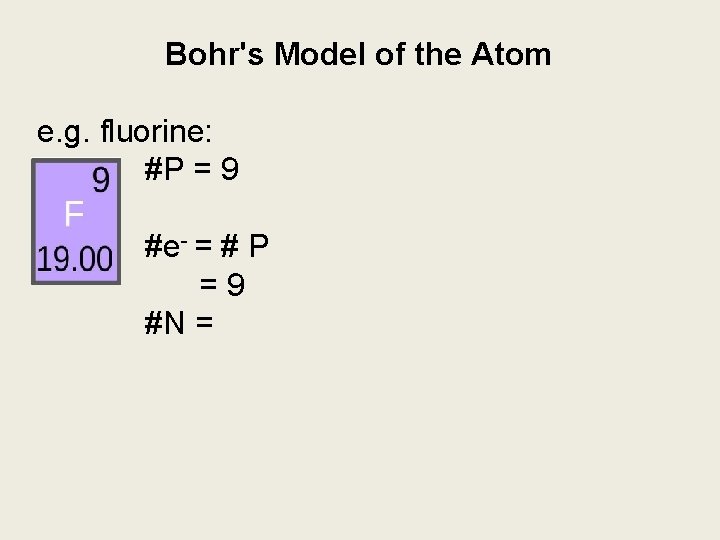
Bohr's Model of the Atom e. g. fluorine: #P = 9 #e- = # P =9 #N =
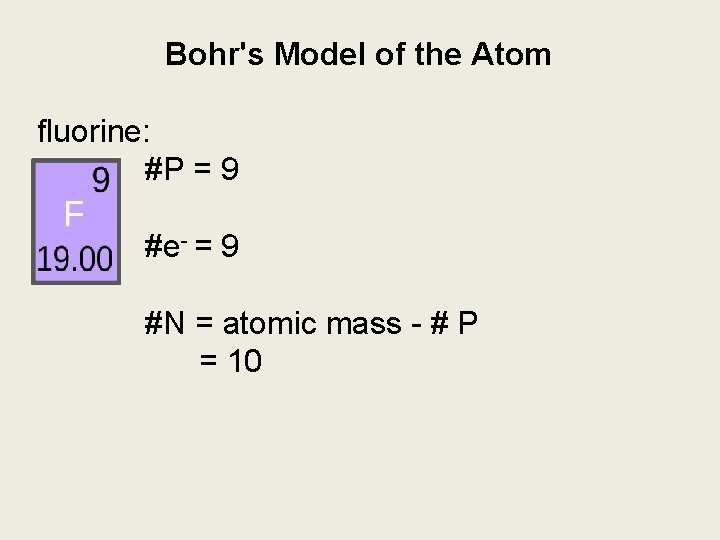
Bohr's Model of the Atom fluorine: #P = 9 #e- = 9 #N = atomic mass - # P = 10
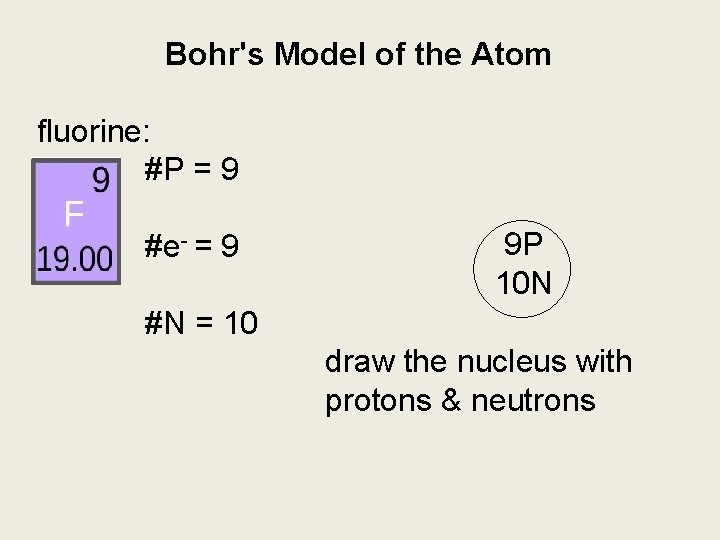
Bohr's Model of the Atom fluorine: #P = 9 #e- = 9 9 P 10 N #N = 10 draw the nucleus with protons & neutrons
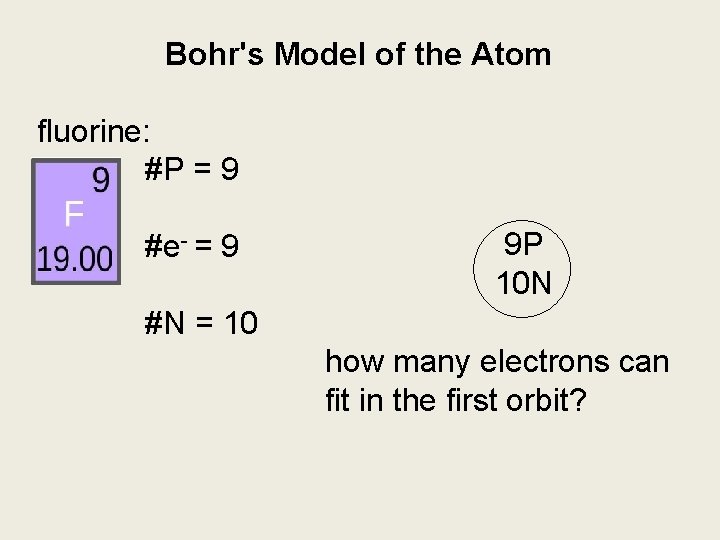
Bohr's Model of the Atom fluorine: #P = 9 #e- = 9 9 P 10 N #N = 10 how many electrons can fit in the first orbit?
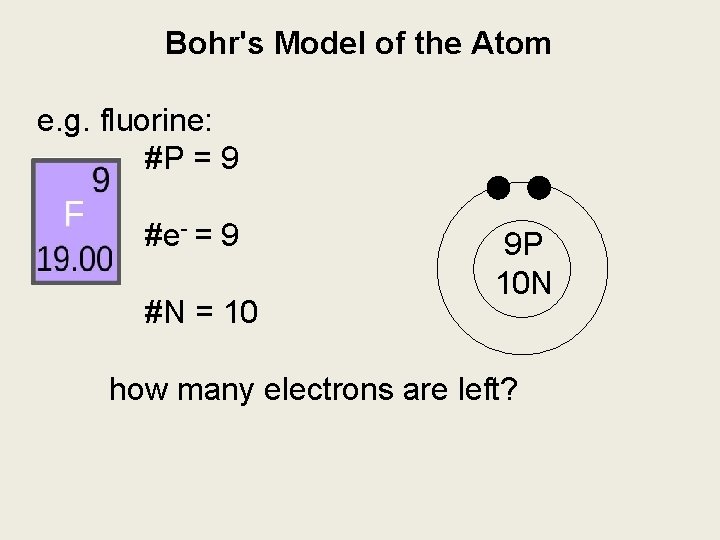
Bohr's Model of the Atom e. g. fluorine: #P = 9 #e- = 9 #N = 10 9 P 10 N how many electrons are left?
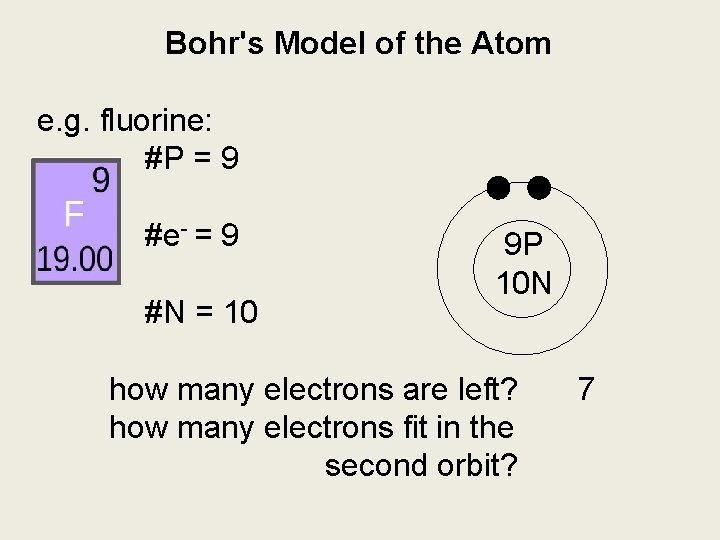
Bohr's Model of the Atom e. g. fluorine: #P = 9 #e- = 9 #N = 10 9 P 10 N how many electrons are left? how many electrons fit in the second orbit? 7
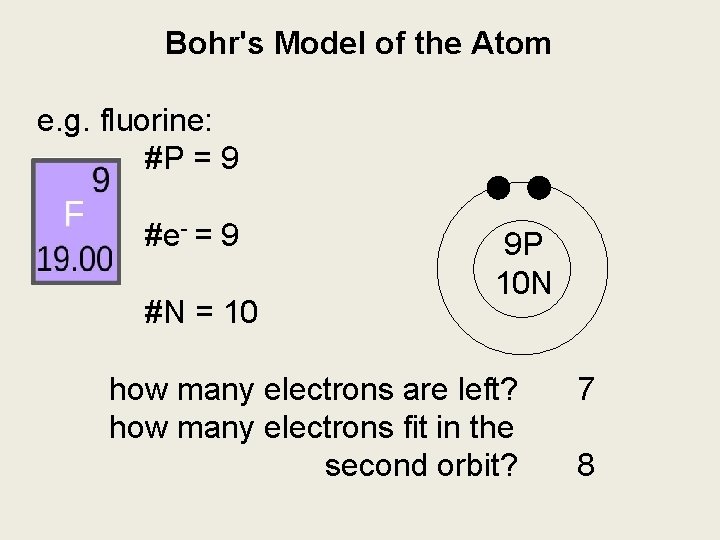
Bohr's Model of the Atom e. g. fluorine: #P = 9 #e- = 9 #N = 10 9 P 10 N how many electrons are left? how many electrons fit in the second orbit? 7 8

Bohr's Model of the Atom e. g. fluorine: #P = 9 #e- = 9 #N = 10 9 P 10 N

Bohr's Model of the Atom try these: hydrogen boron magnesium
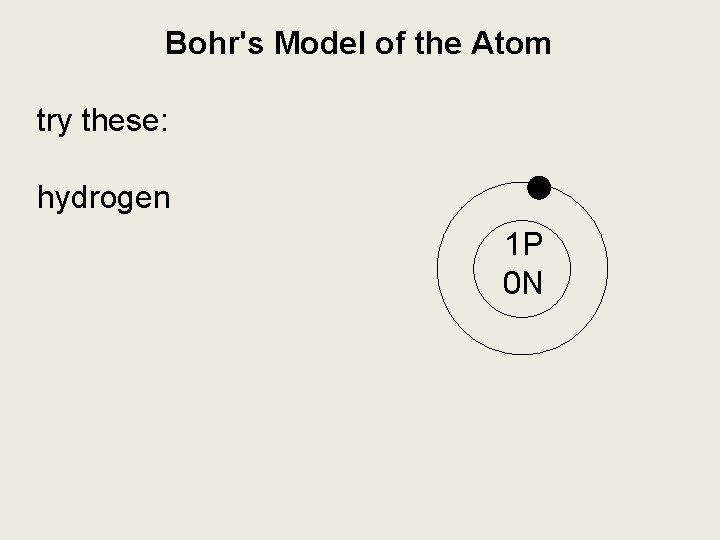
Bohr's Model of the Atom try these: hydrogen 1 P 0 N
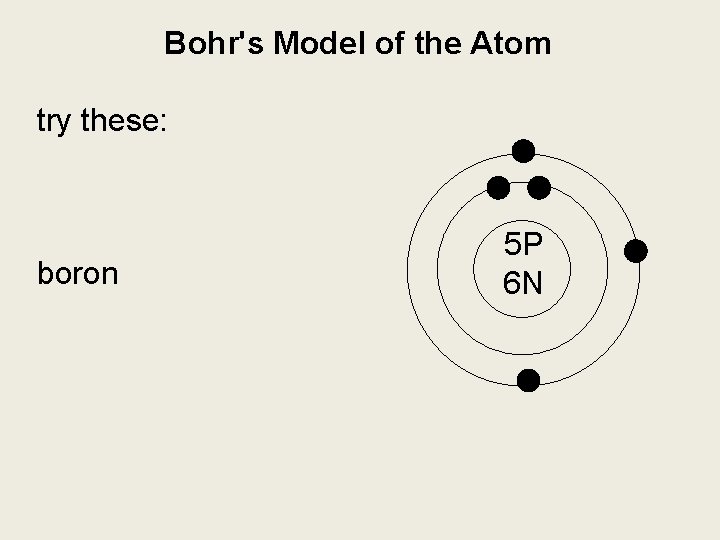
Bohr's Model of the Atom try these: boron 5 P 6 N
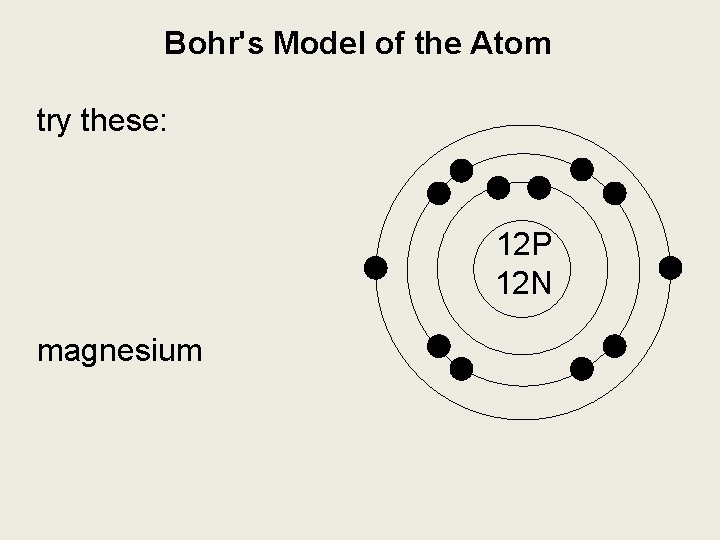
Bohr's Model of the Atom try these: 12 P 12 N magnesium
Activity Draw the 3 different elements using Bohr’s model. Make sure you clearly represent protons, neutrons, and electrons.
- Slides: 18