Bohr Model Structure of an Atom Bohrs Structure

Bohr Model Structure of an Atom

Bohr’s Structure of an Atom • Electrons revolve around the nucleus in different energy levels or shells. • Each shell is associated with a definite amount of energy.

Filling Rules • Maximum number of electrons that can be accommodated in a shell is given by 2 n 2 where n = shell number • For 1 st energy level, n = 1 Maximum number of electrons in 1 st energy level = 2 n 22 x (1) 2 = 2 • For 2 nd energy level n = 2 Maximum number of electrons in the 2 nd energy level = 2 n 22 x 22 = 2 x 4 = 8 • For 3 rd energy level n = 3 Maximum number of electrons in the 3 rd energy level = 2 n 2= 2 x(3) 2= 2 x 9 = 18 • For 4 th energy level n = 4 Maximum number of electrons in the 4 th energy level = 2 n 2= 2 x(4) 2= 2 x 16 = 32
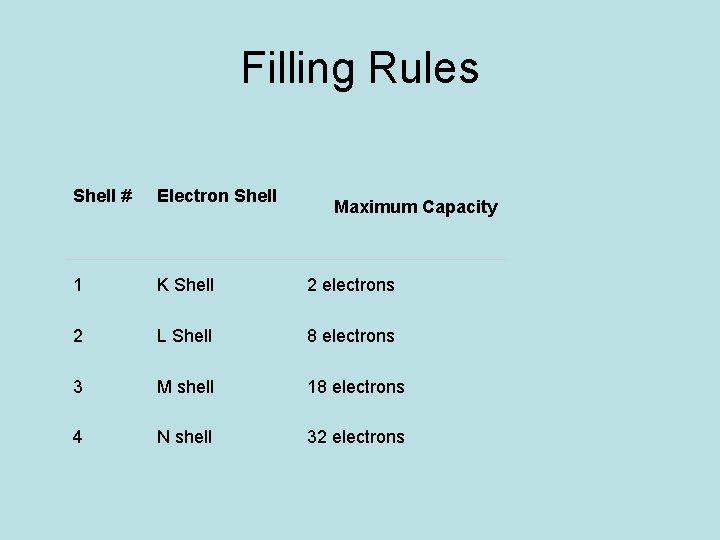
Filling Rules Shell # Electron Shell 1 K Shell 2 electrons 2 L Shell 8 electrons 3 M shell 18 electrons 4 N shell 32 electrons Maximum Capacity

Octet Rule • Atoms tend to combine so that they each have eight elections in their valence shells. • "Rules are made to be broken" -three classes of exceptions to the octet rule ØToo Few Electrons - Electron Deficient Molecules ØToo Many Electrons - Expanded Octets ØLonely Electrons - Free Radicals
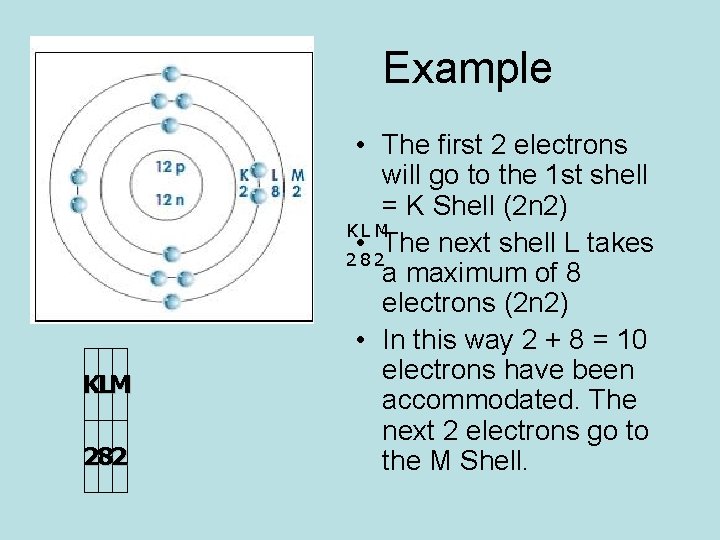
Example KLM 282 • The first 2 electrons will go to the 1 st shell = K Shell (2 n 2) KL M • The next shell L takes 282 a maximum of 8 electrons (2 n 2) • In this way 2 + 8 = 10 electrons have been accommodated. The next 2 electrons go to the M Shell.
- Slides: 6