Bohr Model Review Periods are horizontal rows number
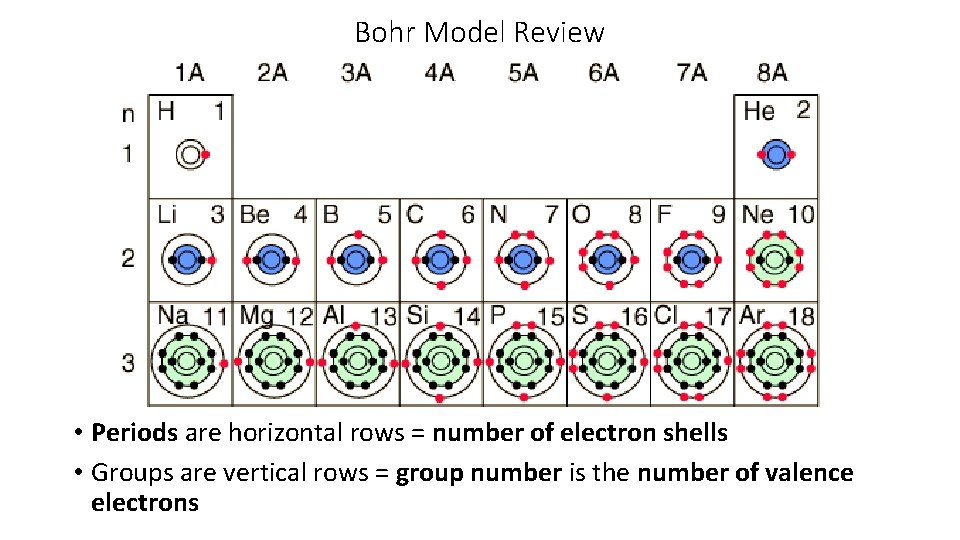
Bohr Model Review • Periods are horizontal rows = number of electron shells • Groups are vertical rows = group number is the number of valence electrons
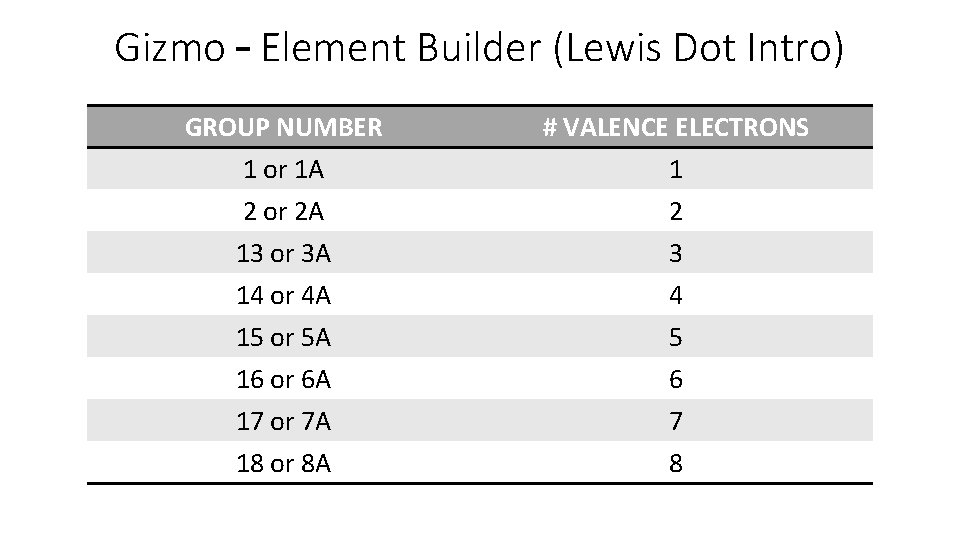
Gizmo – Element Builder (Lewis Dot Intro) GROUP NUMBER 1 or 1 A 2 or 2 A 13 or 3 A 14 or 4 A 15 or 5 A 16 or 6 A 17 or 7 A 18 or 8 A # VALENCE ELECTRONS 1 2 3 4 5 6 7 8
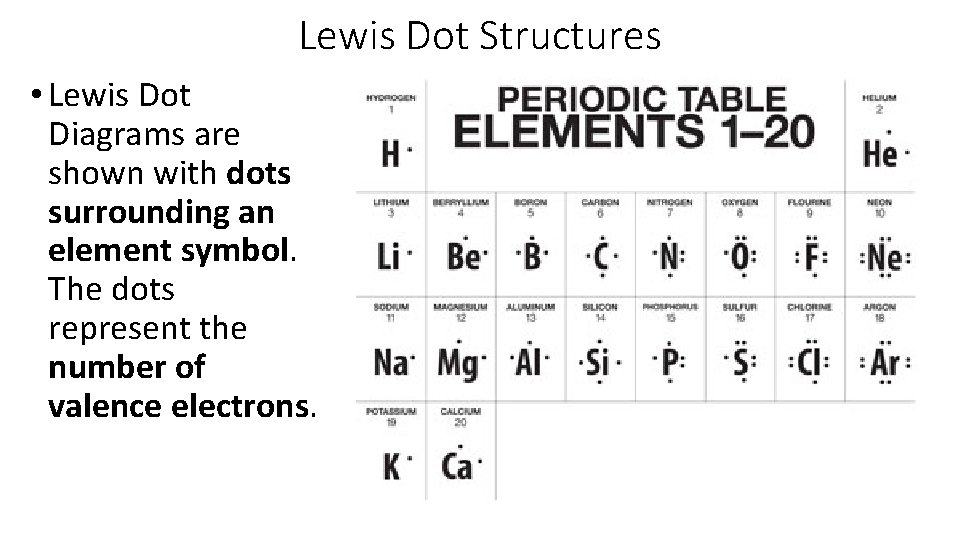
Lewis Dot Structures • Lewis Dot Diagrams are shown with dots surrounding an element symbol. The dots represent the number of valence electrons.
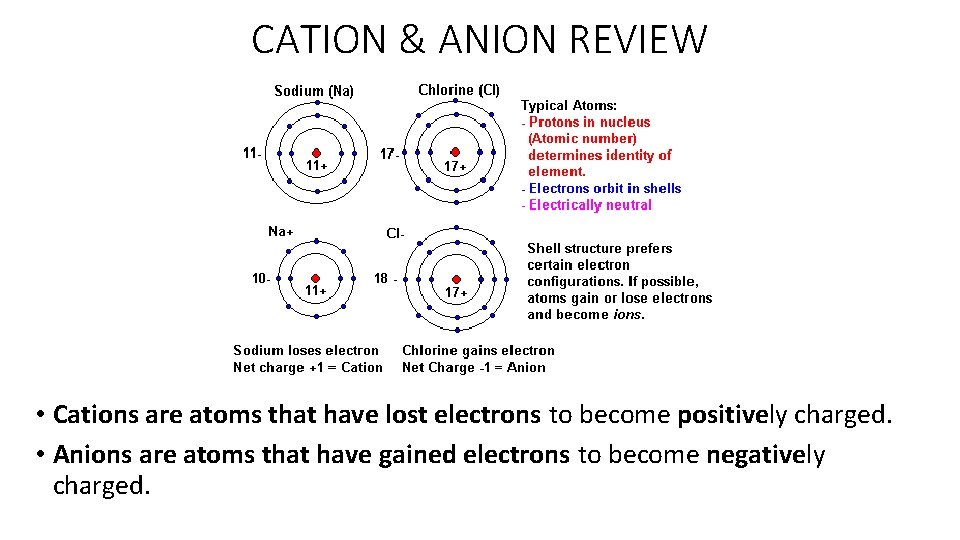
CATION & ANION REVIEW • Cations are atoms that have lost electrons to become positively charged. • Anions are atoms that have gained electrons to become negatively charged.

Metals vs. Nonmetals • Metals want to lose electrons to become cations. • Nonmetals want to gain electrons to become anions.
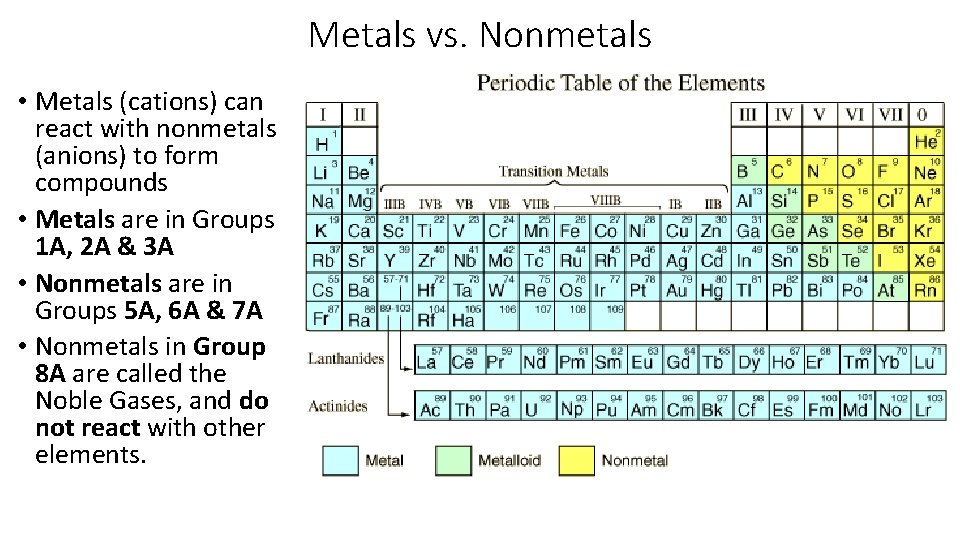
Metals vs. Nonmetals • Metals (cations) can react with nonmetals (anions) to form compounds • Metals are in Groups 1 A, 2 A & 3 A • Nonmetals are in Groups 5 A, 6 A & 7 A • Nonmetals in Group 8 A are called the Noble Gases, and do not react with other elements.

The Octet Rule • https: //www. youtube. com/watch? v=4 OKy 782 e. PKM • Elements in Group 1 A, 2 A and 3 A want to lose electrons to achieve an outermost shell of 2 (Lithium and Beryllium only) or 8 valence electrons. Elements in Groups 1 A – 2 A become cations. • Elements in Group 1 A want to lose 1 electron • Elements in Group 2 A want to lose 2 electrons • Elements in Group 3 A want to lose 3 electrons • Elements in Group 5 A, 6 A, 7 A want to gain electrons to achieve an outermost shell of 8 valence electrons. Elements in Groups 5 A – 7 A become anions. • Elements in Group 5 A want to gain 3 electrons • Elements in Group 6 A want to gain 2 electrons • Elements in Group 7 A want to gain 1 electron
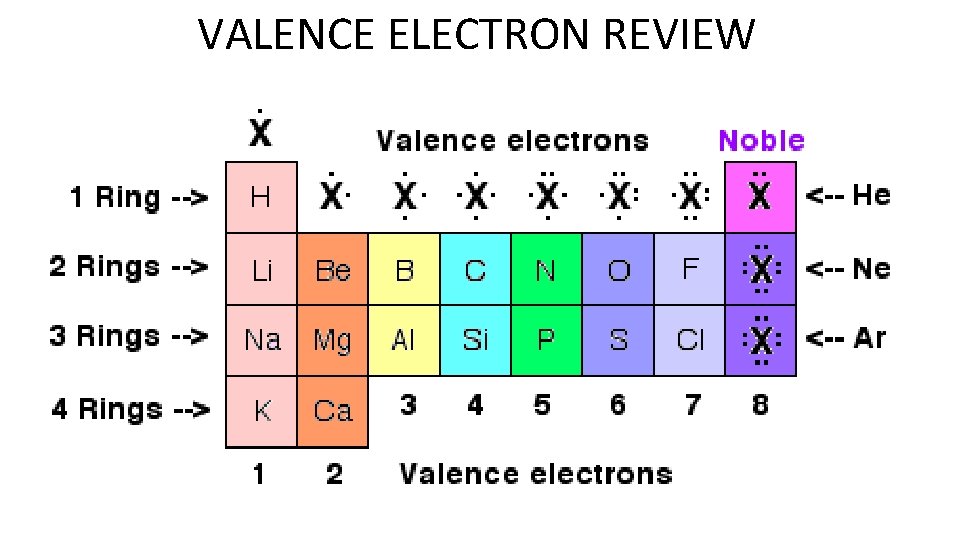
VALENCE ELECTRON REVIEW
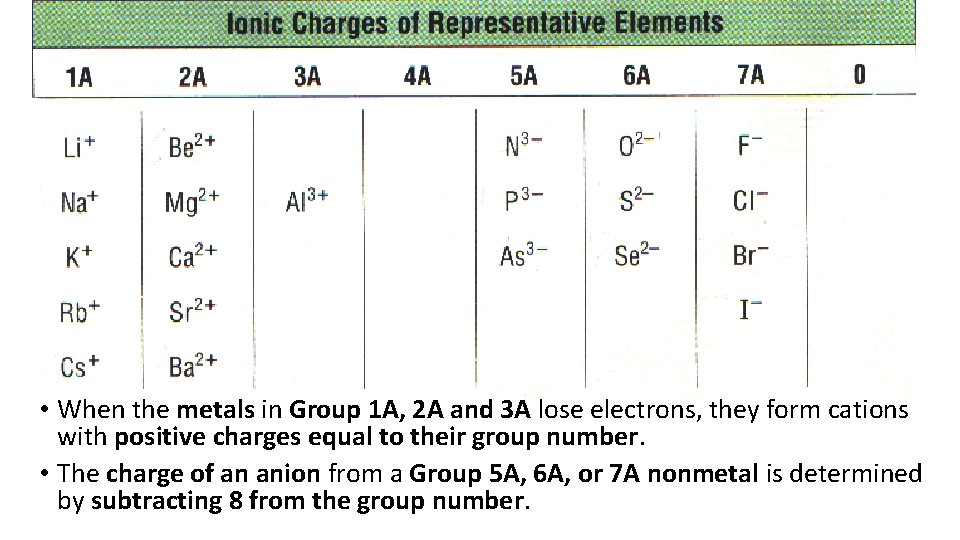
• When the metals in Group 1 A, 2 A and 3 A lose electrons, they form cations with positive charges equal to their group number. • The charge of an anion from a Group 5 A, 6 A, or 7 A nonmetal is determined by subtracting 8 from the group number.

Lewis Model of Bonding • For ionic bonds, this model shows the transfer of electrons from cations to anions. • Cations will lose all their valence electrons, and anions will gain electrons to have a full shell (TOTAL OF 8 ELECTRONS).

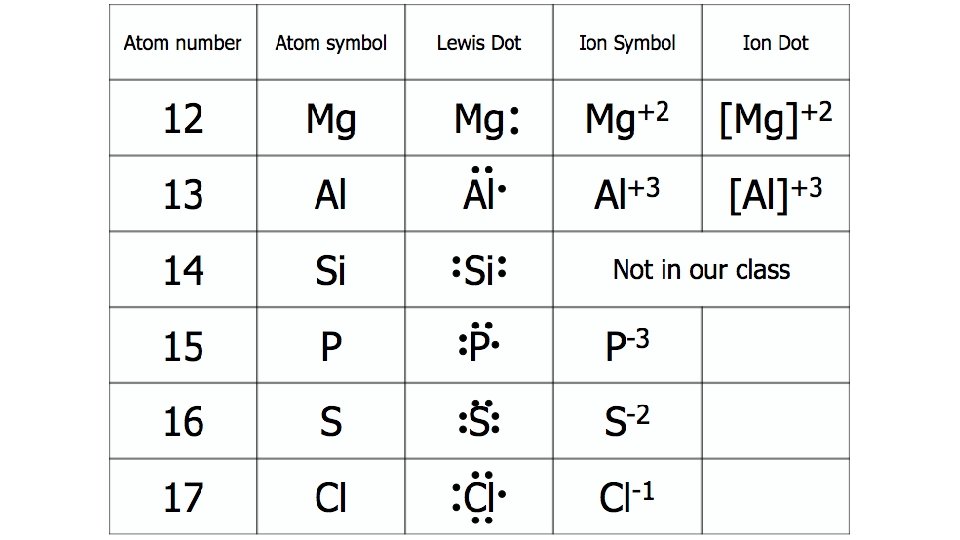
Example Problems
- Slides: 12