Bohr Model Bohr diagrams show many electrons appear
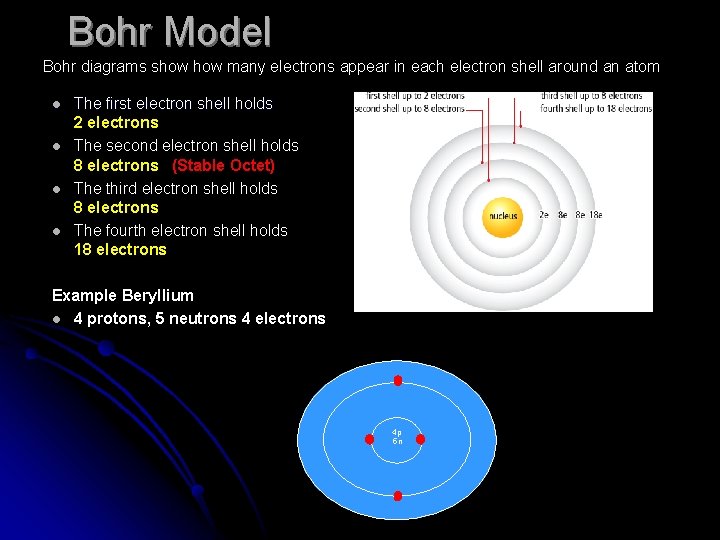
Bohr Model Bohr diagrams show many electrons appear in each electron shell around an atom l l The first electron shell holds 2 electrons The second electron shell holds 8 electrons (Stable Octet) The third electron shell holds 8 electrons The fourth electron shell holds 18 electrons Example Beryllium l 4 protons, 5 neutrons 4 electrons 4 p 5 n
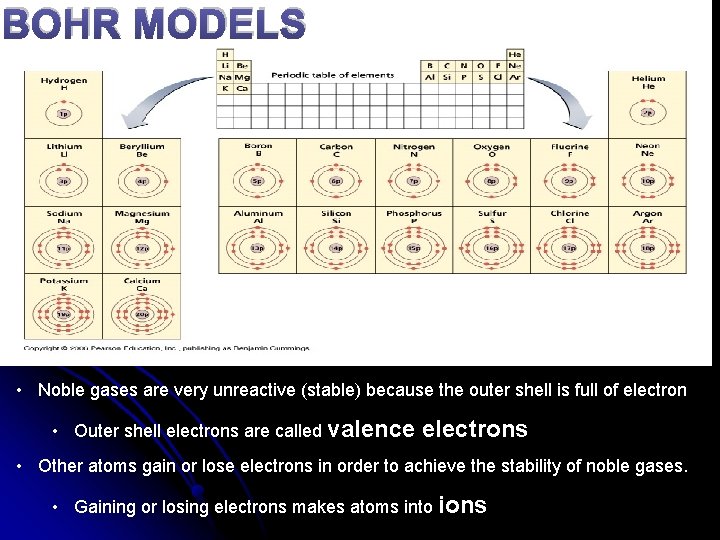
BOHR MODELS • Noble gases are very unreactive (stable) because the outer shell is full of electron • Outer shell electrons are called valence electrons • Other atoms gain or lose electrons in order to achieve the stability of noble gases. • Gaining or losing electrons makes atoms into ions

Ion Formation RECAP l Metals tend to lose electrons and written: l l l Na+1, Be 2+ Positive ions are called CATIONS Non-Metals tend to gain electrons l O 2 -, F 1 - l Negative ions are called ANIONS
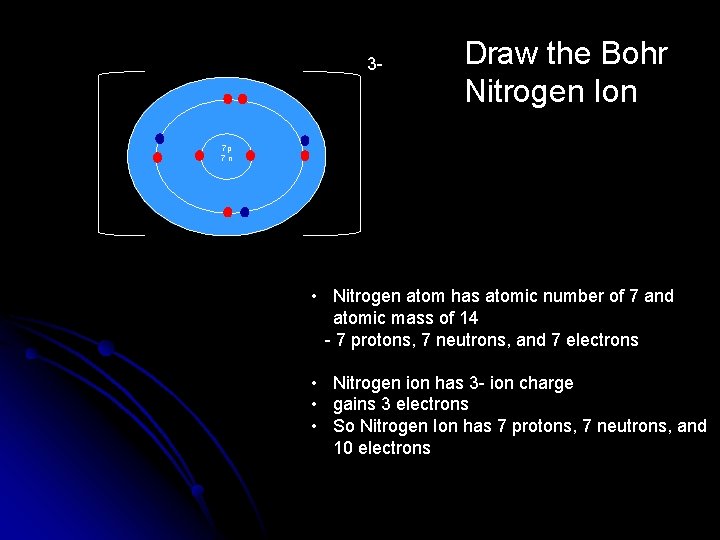
3 - Draw the Bohr Nitrogen Ion 7 p 7 n • Nitrogen atom has atomic number of 7 and atomic mass of 14 - 7 protons, 7 neutrons, and 7 electrons • Nitrogen ion has 3 - ion charge • gains 3 electrons • So Nitrogen Ion has 7 protons, 7 neutrons, and 10 electrons
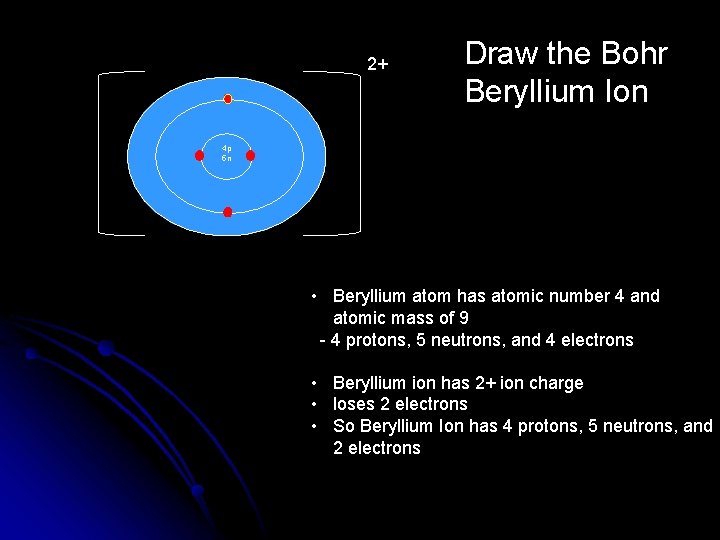
2+ Draw the Bohr Beryllium Ion 4 p 5 n • Beryllium atom has atomic number 4 and atomic mass of 9 - 4 protons, 5 neutrons, and 4 electrons • Beryllium ion has 2+ ion charge • loses 2 electrons • So Beryllium Ion has 4 protons, 5 neutrons, and 2 electrons
- Slides: 6