BLOOD PRODUCT TRANSFUSIONTRANSFUSION REACTION D45 Nursing Policy Procedure
![BLOOD PRODUCT TRANSFUSION/TRANSFUSION REACTION [D-45] Nursing Policy & Procedure Manual 11/2014 Policy Review & BLOOD PRODUCT TRANSFUSION/TRANSFUSION REACTION [D-45] Nursing Policy & Procedure Manual 11/2014 Policy Review &](https://slidetodoc.com/presentation_image_h2/85abd9e207610e679542e5e0e9beb8cb/image-1.jpg)
BLOOD PRODUCT TRANSFUSION/TRANSFUSION REACTION [D-45] Nursing Policy & Procedure Manual 11/2014 Policy Review & Revision

Directions for Completion of this program awards 0. 25 CE for nurses. 1. Before proceeding to the posttest, be sure you have completed the review of the all slides/information. 2. The posttest is final step of this education. � � � Remember, no attendance record is needed. Completion of the posttest will be sent electronically to your Edu. Tracker record once a 100% is achieved. Print the Certificate of Completion for your records if desired.

Why? Recent chart reviews have revealed inconsistencies with practice expectations and policy requirements related to blood product transfusions. These inconsistencies can lead to serious patient events and/or citations from regulatory bodies, including The Joint Commission or Department of Health. Patient events related to blood product transfusions can result in serious harm and death. SLUHN goal = avoid inconsistencies and patient harm!

Program Objectives • Review key SLUHN policy requirements identified in the Blood Product Transfusion/Transfusion Reaction policy. • Clarify key SLUHN practice expectations regarding blood product transfusions.

Orders… • A specific order for transfusion of blood components must be entered before a computer request is sent to the Blood Bank. • In emergency situations, a “verbal” order for administration of blood products may be accepted by the RN (order is entered as soon as possible following the emergent situation). • NOTE: Consent will be obtained by the patient’s physician or advanced practitioner as defined in the Administrative Policy for Informed Consent.

Type and Crossmatch… • A type and crossmatch is valid for 3 days. • Blood products will not be issued from the Blood Bank without an order for full crossmatch and an order to transfuse. • In an emergency, uncrossmatched blood will be dispensed with the physician making the decision and assuming full responsibility. If blood is not crossmatched, the Emergency Transfusion Request form will be completed.

Blood Products Transported out of the Blood Bank… • Once a blood product is received in the patient care area, the product should be inspected and administered as soon as possible. • Transfusion of blood products must be initiated within 30 minutes of issue from the Blood Bank. • If this is not possible, the blood product must be returned to the Blood Bank within the 30 minutes. NEVER store blood products in the refrigerator on the unit.

Blood Products Transported out of the Blood Bank… • Exception to the 30 minute rule: Multiple units may be stored in a Blood Bank cooler for emergency situations (e. g. trauma, code crimson, post cardiothoracic surgery, OR). Blood products will generally maintain acceptable temperature for up to 6 hours under normal room temperature conditions if kept in the closed Blood Bank-issued cooler with an ice pack or wet ice. Coolers are returned to the Blood Bank with unused blood products at 6 hours and can be repackaged if necessary.
Patient Identification… • Positive patient identification is of the utmost importance in blood product transfusions. • Blood products will not be issued without printed confirmation of the patient’s name and identification number. • The Blood Product Issue Request form should be completed by the patient care staff and include the required information. • Verbal request is acceptable from the OR or in an emergency situation. Patient name and medical record number must be provided if available.

Patient Identification… • Before initiating a blood or blood component transfusion, the patient is objectively matched to the blood or blood component during a two-person bed-side or chair-side verification process. • When using a two-person bed-side or chair-side verification process, one individual conducting the identification verification must be the qualified transfusionist who will administer the blood or blood component to the patient. • When using a two-person bed-side or chair-side verification process, the second individual conducting the identification verification must be qualified to participate in the process. • FYI… A Blood Product Verification Process educational program is available on Tracker Trainer and a SLUHN competency is available for those who require and/or need to learn this skill.

Practice Reminder… WITNESS Verification of Blood Product Labeling: • • When the transfusionist reads each of the items on the unit bag label and unit tag the witness verifies the information on the transfusion record. When the witness reads each of the items on the transfusion record the transfusionist verifies the information on the unit bag label and unit tag. TRANSFUSIONIST Information verified

• Baseline vital signs must be taken prior to obtaining blood products. If the patient’s temperature is ≥ 101°F/38. 5°C notify the physician/AP. • Obtain and document vital signs (on the transfusion record) at the initiation, 15 minutes after starting, and conclusion of the transfusion. • This means three sets of vital signs are documented on the transfusion record. • The vital signs need to be documented on the transfusion record even if they are documented in the patient’s medical record (i. e. electronically). • Intra-operatively, documentation of vital signs may be recorded on the Anesthesia Record.
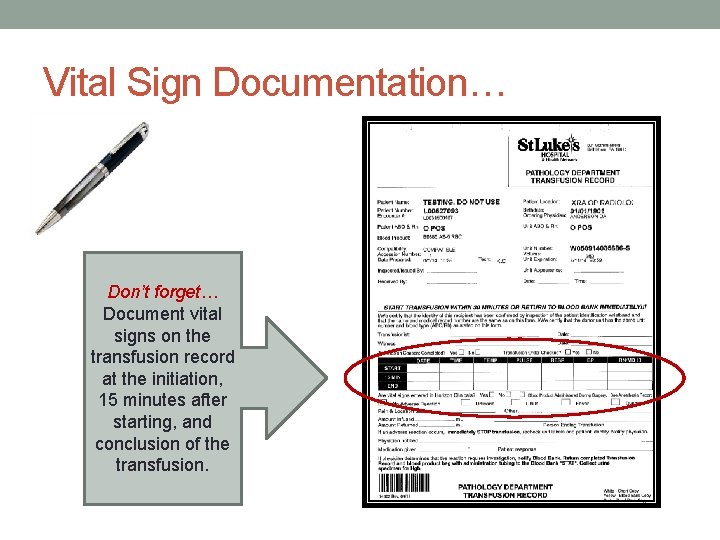
Vital Sign Documentation… Don’t forget… Document vital signs on the transfusion record at the initiation, 15 minutes after starting, and conclusion of the transfusion.
Assessment During Transfusion… • The RN or other transfusionist assesses the patient for signs/symptoms of a transfusion reaction throughout the duration of the transfusion. • Generally, the assessments occur at 5 minutes after the start of the transfusion, then 15, 30, 60 minutes and every 60 minutes thereafter until the transfusion is complete. • The RN or other transfusionist documents assessment findings that are abnormal. • Abnormal findings indicate a transfusion reaction which would be documented on the transfusion record in addition to the documentation in the patient’s medical record (i. e. electronically).
Transfusion Reaction… Nurses and transfusionists administering blood components should be well aware of the signs and symptoms of a possible reaction and be prepared to take steps to mitigate the current episode as well as prevent future similar reactions when possible.

Signs & Symptoms of a Transfusion Reaction Signs & symptoms include the following but this is not an all inclusive list… • Fever • Chills • Respiratory distress • Hypertension/hypotension • Abdominal, chest, flank, or back pain • Pain at the infusion site • Skin manifestations, including urticarial, rash, flushing, pruritis, and localized edema • Jaundice or hemoglobinuria • Nausea/vomiting • Abnormal bleeding • Oliguria/anuria Please see Blood Product Transfusion/Transfusion Reaction [D-45] policy in the online Nursing Policy & Procedure manual for additional information.
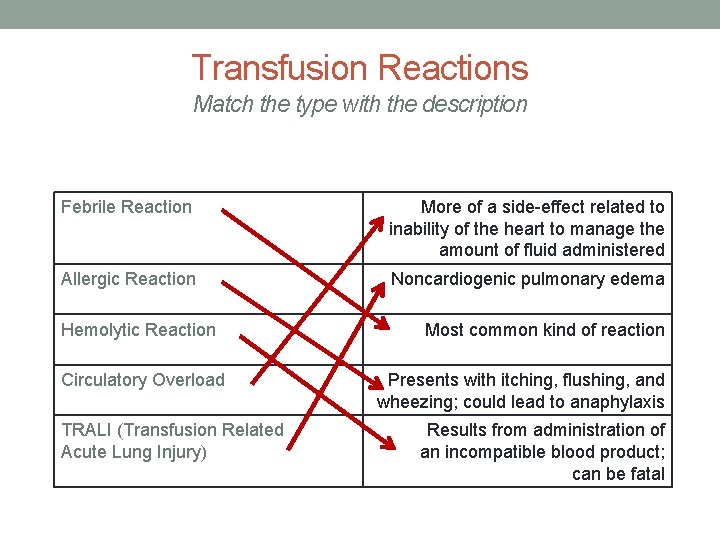
Transfusion Reactions Match the type with the description Febrile Reaction More of a side-effect related to inability of the heart to manage the amount of fluid administered Allergic Reaction Noncardiogenic pulmonary edema Hemolytic Reaction Circulatory Overload TRALI (Transfusion Related Acute Lung Injury) Most common kind of reaction Presents with itching, flushing, and wheezing; could lead to anaphylaxis Results from administration of an incompatible blood product; can be fatal

Transfusion Reaction Documentation… Don’t forget… Document a transfusion reaction on the transfusion record; additional information is documented in the patient’s medical record (i. e. electronically).

Documentation… • Complete the transfusion record for each unit (blood component) administered. • Note: Intra-operatively, after the two person verification is documented, remaining documentation may be recorded on the Anesthesia Record. • Record adverse reaction on the lower portion of the transfusion record, if indicated. • Place white (paper) copy of the transfusion record on the patient’s chart; forward the yellow copy to the Blood Bank. • If blood products are released from the Blood Bank in a cooler and blood products are administered, a reconciled copy of the Laboratory blood product log (e. g. Surgical Procedure Blood Products log) will be sent to the patient care area and placed in the patient’s medical record.

Policy Release & Implementation Planned for week of December 15, 2014

THANK YOU FOR YOUR ACCOUNTABILITY TO PROVIDE SAFE PATIENT CARE RELATED TO BLOOD PRODUCT TRANSFUSIONS Please proceed to the program posttest. Print the certificate of completion for your records if desired.
- Slides: 21