Blood Gases p H and Buffer system Part

Blood Gases, p. H and Buffer system Part 2 Lecture 17 Dr. Mazen Alzaharna CC 2018/1
Oxygen • Role of O 2 in metabolism is crucial to all life • Synthesis of ATP from ADP • Evaluation of patient’s oxygen status is possible using partial pressure of oxygen (PO 2) measured along with PCO 2 and p. H in blood gas analysis • In patients with respiratory disorders, a disturbance of the partial pressure of oxygen (PO 2) may be of greater clinical significance than either an abnormal PCO 2 or abnormal [H+]. Dr. Mazen Alzaharna CC 2018/1 2
Tissue oxygenation For adequate tissue oxygenation the following conditions are necessary: • 1. Available atmospheric O 2 2. Adequate ventilation 3. Gas exchange is sufficient (lung & arterial blood) 4. Loading of O 2 onto Hemoglobin 5. Adequate Hemoglobin 6. Adequate transport and release mechanism of O 2 is properly working. • Any disturbances in these conditions can result in poor tissue oxygenation Dr. Mazen Alzaharna CC 2018/1 3
Oxygen Transport • • Most of the O 2 in arterial blood is transported to the tissues by Hb. The amount of O 2 loaded onto Hb depends on the following: 1. 2. 3. 4. 5. 6. 7. • Availability of O 2. Concentration and types of Hb present. Presence of nonoxygen substances such as CO. H+ (p. H). Blood temperature. p. CO 2 levels. 2, 3 -DPG levels. With normal diffusion of O 2 to the arterial blood, more than 95% of the functional Hb (hemoglobin capable of reversibly binding O 2 ) will bind O 2. Dr. Mazen Alzaharna CC 2018/1 4
Blood Hb • Blood Hb exists in one of four conditions: 1. Oxyhemoglobin (O 2 Hb), O 2 is reversibly bound to Hb. 2. Deoxyhemoglobin (HHb), Hb not bound to O 2, but is capable of forming a bond when O 2 is available. 3. Carboxyhemoglobin (COHb), the bond between CO and Hb is reversible, • but is > 200 times as strong as the bond between O 2 and Hb. 4. Methemoglobin (Met-Hb), Hb unable to bind O 2 because Fe is in the oxidized state. • Fe+3 can be reduced by the enzyme met. Hb-reductase which is found in RBCs. Dr. Mazen Alzaharna CC 2018/1 5
Hemoglobin-Oxygen dissociation • O 2 dissociates from Adult hemoglobin (Hb-A) readily. • Hb holds on the O 2 until O 2 tension is reduced to 60 mm. Hg- then O 2 released rapidly. • In tissue, exposure to elevated CO 2 and H+ results in enhanced O 2 release. • This release of oxygen from hemoglobin accelerates the uptake of CO 2 and H+ by hemoglobin (acid-base buffering). • In the lungs, the microenvironment promotes uptake of O 2 and release of CO 2. Dr. Mazen Alzaharna CC 2018/1 6
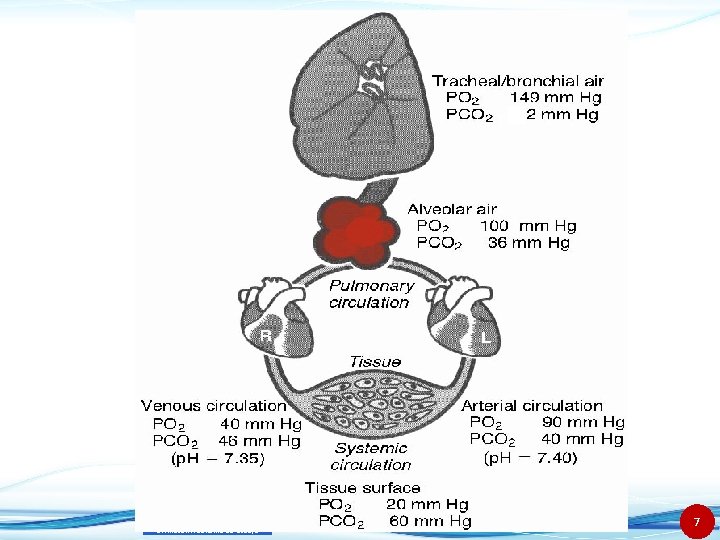
Dr. Mazen Alzaharna CC 2018/1 7
Hemoglobin-Oxygen dissociation • In addition to adequate ventilation and gas exchange with the pulmonary circulation, O 2 must be released at the tissues. • Hemoglobin transports O 2. • The increased H+ concentration and p. CO 2 levels at the tissue from cellular metabolism change the molecular configuration of O 2 Hb, facilitating O 2 release. Dr. Mazen Alzaharna CC 2018/1 8
Hemoglobin-Oxygen dissociation • Oxygen dissociates from adult hemoglobin (A 1) in a characteristic fashion. • If this dissociation is graphed with p. O 2 on the x-axis and percent SO 2 on the y-axis, the resulting curve is slightly S shaped. • Hemoglobin “holds on” to O 2 until the O 2 tension in the tissue is reduced to about 60 mm Hg. • Below this tension, the O 2 is released rapidly. • The position of the oxygen dissociation curve reflects the affinity that hemoglobin has for O 2 and affects the rate of this dissociation. Dr. Mazen Alzaharna CC 2018/1 9
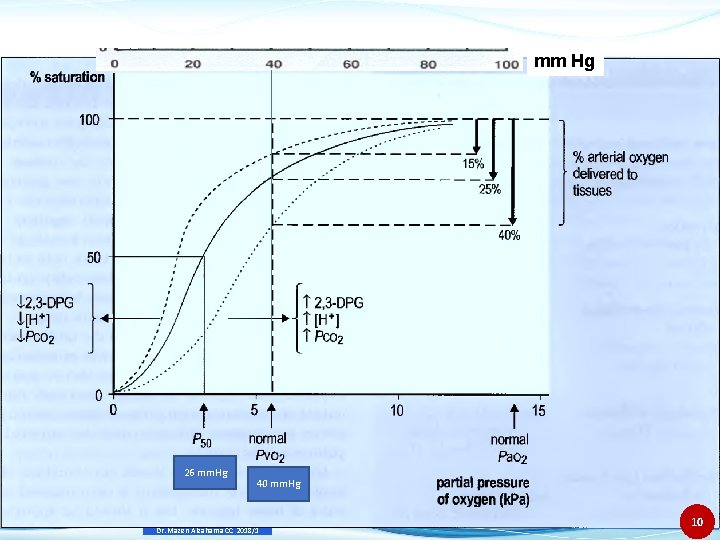
mm Hg 26 mm. Hg 40 mm. Hg Dr. Mazen Alzaharna CC 2018/1 10
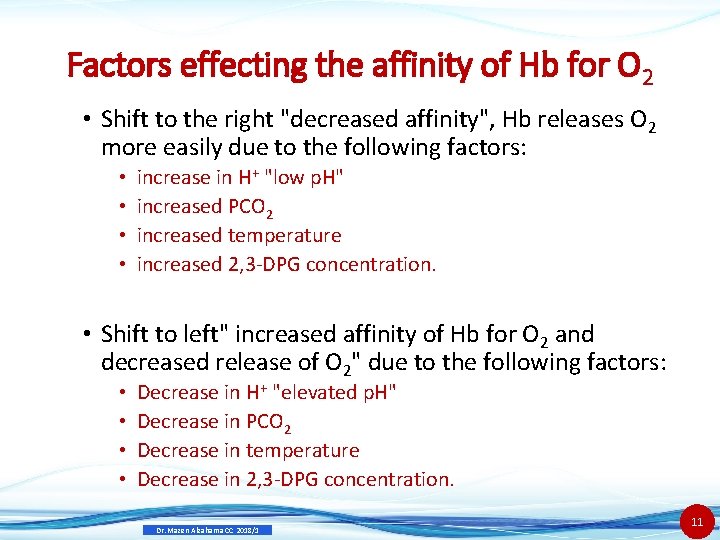
Factors effecting the affinity of Hb for O 2 • Shift to the right "decreased affinity", Hb releases O 2 more easily due to the following factors: • • increase in H+ "low p. H" increased PCO 2 increased temperature increased 2, 3 -DPG concentration. • Shift to left" increased affinity of Hb for O 2 and decreased release of O 2" due to the following factors: • • Decrease in H+ "elevated p. H" Decrease in PCO 2 Decrease in temperature Decrease in 2, 3 -DPG concentration. Dr. Mazen Alzaharna CC 2018/1 11
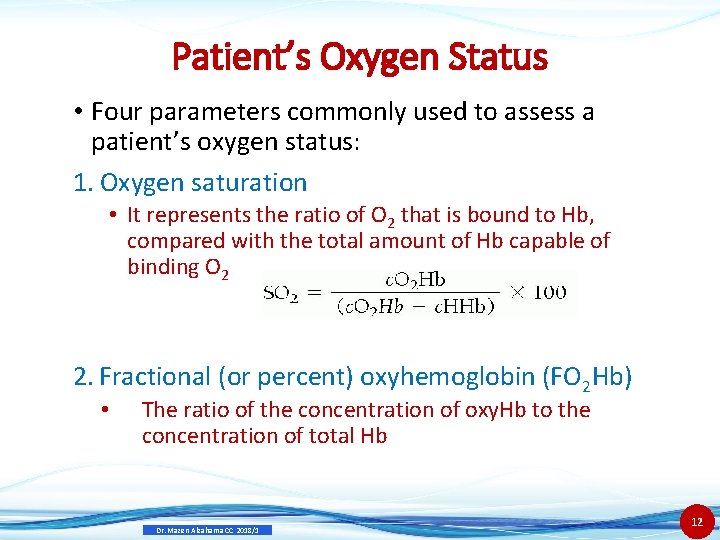
Patient’s Oxygen Status • Four parameters commonly used to assess a patient’s oxygen status: 1. Oxygen saturation • It represents the ratio of O 2 that is bound to Hb, compared with the total amount of Hb capable of binding O 2 2. Fractional (or percent) oxyhemoglobin (FO 2 Hb) • The ratio of the concentration of oxy. Hb to the concentration of total Hb Dr. Mazen Alzaharna CC 2018/1 12
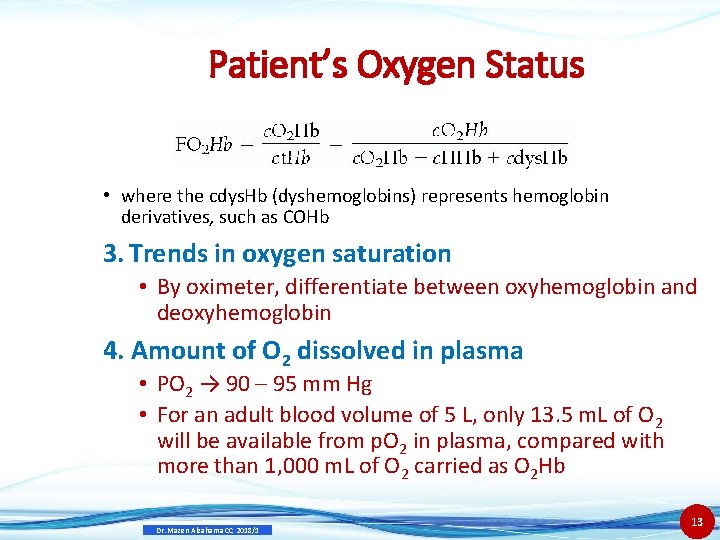
Patient’s Oxygen Status • where the cdys. Hb (dyshemoglobins) represents hemoglobin derivatives, such as COHb 3. Trends in oxygen saturation • By oximeter, differentiate between oxyhemoglobin and deoxyhemoglobin 4. Amount of O 2 dissolved in plasma • PO 2 → 90 – 95 mm Hg • For an adult blood volume of 5 L, only 13. 5 m. L of O 2 will be available from p. O 2 in plasma, compared with more than 1, 000 m. L of O 2 carried as O 2 Hb Dr. Mazen Alzaharna CC 2018/1 13

Collection and Handling of Specimen • The specimen for blood gases and p. H should be arterial blood collected in heparinized plastic containers including syringes • All air bubbles should be removed before mixing the specimen, • The needle replaced or the ends of the capillary tube fitted with a tight-fitting cover Dr. Mazen Alzaharna CC 2018/1 14
Collection and Handling of Specimen • The specimen must be placed in ice water until analysis, unless it is analyzed immediately at the patient’s bedside. • Temperatures warmer than 4 o. C allow: • cell glycolysis to continue in the whole blood specimen, • resulting in falsely decreased p. H • and decreased partial pressure of oxygen (PO 2) • and falsely increased partial pressure of carbon dioxide (PCO 2). Dr. Mazen Alzaharna CC 2018/1 15
Pulse Oximetry • Noninvasive measurements for following “trends” in oxygenation are attained with pulse oximetry (Sp. O 2). • These devices pass light of two or more wavelengths through the tissue in the capillary bed of the toe, finger, or ear. • Until recently, pulse oximetry technology could not measure COHB and Met. Hb. • For those pulse oximeters that calculate oxy. Hb saturation based only on oxy. Hb and deoxy. Hb, oxy. Hb saturation will be overestimated when one or more dyshemoglobins are present. • In addition, the accuracy of pulse oximetry can be compromised by many factors, including poor perfusion and severe anemia. Dr. Mazen Alzaharna CC 2018/1 16
Measurements Spectrophotometric ( co-oximeter) • Co-oximeter designed to determine various Hb species directly • Each species has a characteristic absorbance curve • Instruments should have 4 W. L. for: • HHb, O 2 Hb, COHb and Met. Hb Dr. Mazen Alzaharna CC 2018/1 17
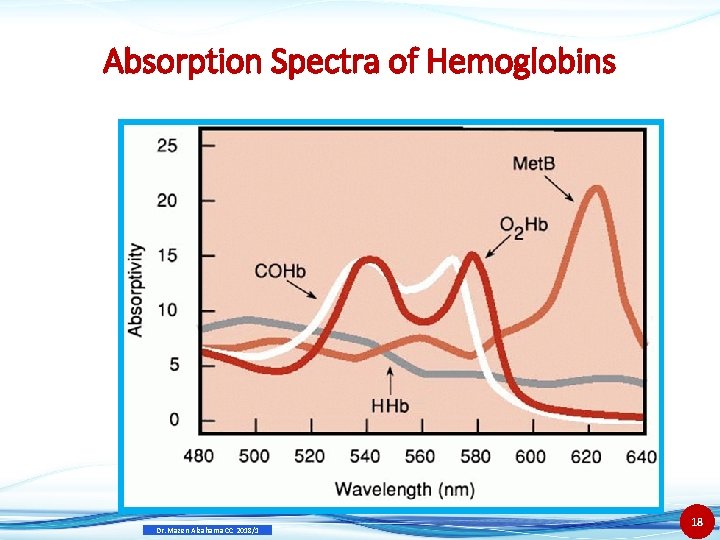
Absorption Spectra of Hemoglobins Dr. Mazen Alzaharna CC 2018/1 18
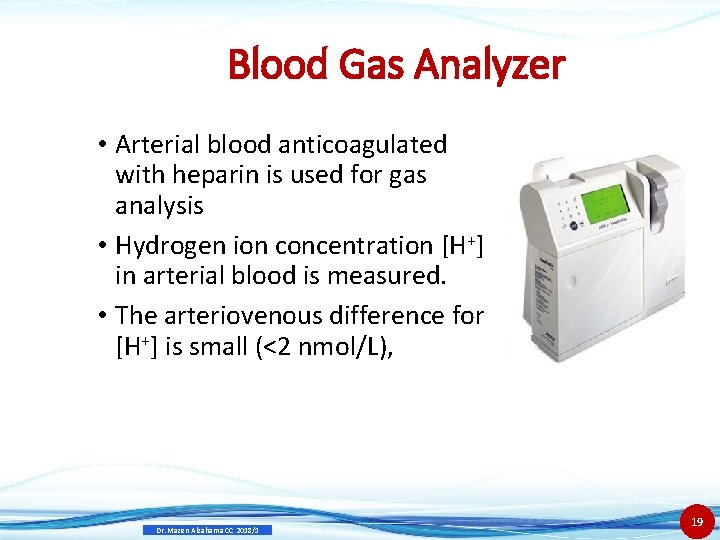
Blood Gas Analyzer • Arterial blood anticoagulated with heparin is used for gas analysis • Hydrogen ion concentration [H+] in arterial blood is measured. • The arteriovenous difference for [H+] is small (<2 nmol/L), Dr. Mazen Alzaharna CC 2018/1 19

Blood Gas Analyzer • but the difference is significant for PCO 2 approximately 6 mm. Hg higher in venous blood. • and PO 2, 50 mm. Hg lower in venous blood. • Use electrode method for sensing and measuring: • PO 2 • PCO 2 • p. H Dr. Mazen Alzaharna CC 2018/1 20
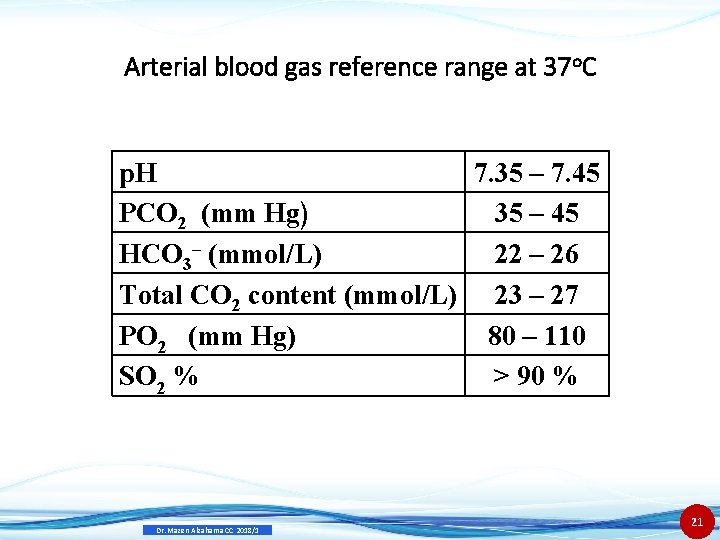
Arterial blood gas reference range at 37 o. C p. H 7. 35 – 7. 45 PCO 2 (mm Hg) 35 – 45 HCO 3– (mmol/L) 22 – 26 Total CO 2 content (mmol/L) 23 – 27 PO 2 (mm Hg) 80 – 110 SO 2 % > 90 % Dr. Mazen Alzaharna CC 2018/1 21
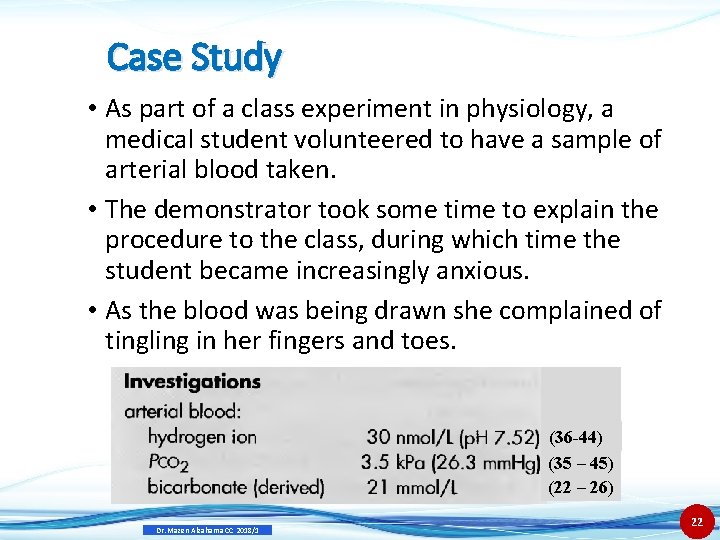
Case Study • As part of a class experiment in physiology, a medical student volunteered to have a sample of arterial blood taken. • The demonstrator took some time to explain the procedure to the class, during which time the student became increasingly anxious. • As the blood was being drawn she complained of tingling in her fingers and toes. c (36 -44) (35 – 45) (22 – 26) Dr. Mazen Alzaharna CC 2018/1 22
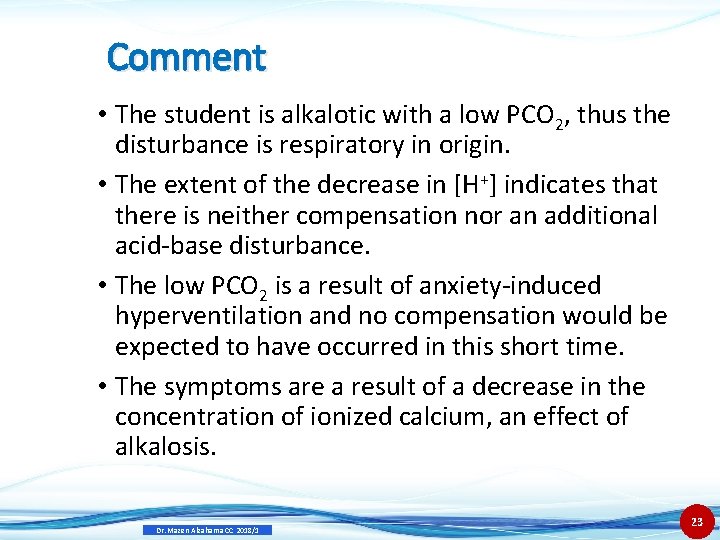
Comment • The student is alkalotic with a low PCO 2, thus the disturbance is respiratory in origin. • The extent of the decrease in [H+] indicates that there is neither compensation nor an additional acid-base disturbance. • The low PCO 2 is a result of anxiety-induced hyperventilation and no compensation would be expected to have occurred in this short time. • The symptoms are a result of a decrease in the concentration of ionized calcium, an effect of alkalosis. Dr. Mazen Alzaharna CC 2018/1 23

Case Study • A young woman was admitted to hospital 8 h after she had taken an overdose of aspirin. (36 -44) (35 – 45) Dr. Mazen Alzaharna CC 2018/1 24

Comment • There is an acute respiratory alkalosis with a coexistent non -respiratory acidosis. • This combination is characteristic of salicylate poisoning, where initial respiratory stimulation causes a respiratory alkalosis but later the metabolic effects of salicylate tend to predominate, producing an acidosis. • This case history illustrates the importance of considering the clinical setting when analyzing acid-base data. • Calculation of the anion gap might have been helpful here. • It would have been increased by the presence of organic anions, indicating a coexisting non-respiratory acidosis, but is normal in respiratory alkalosis. Dr. Mazen Alzaharna CC 2018/1 25
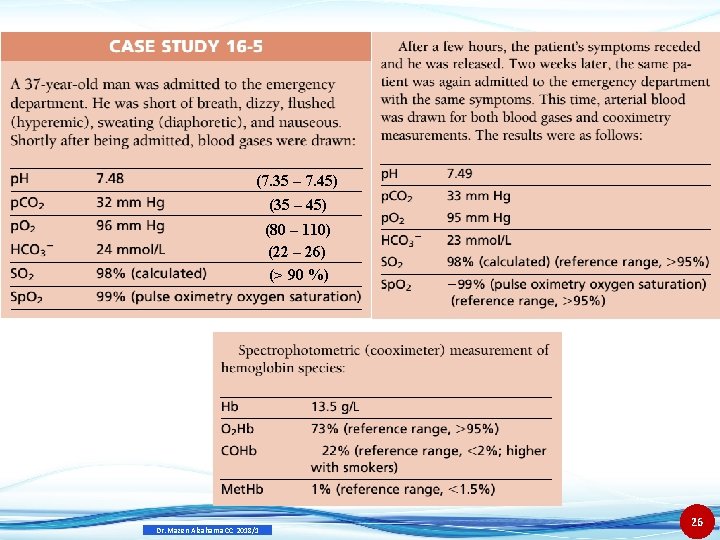
(7. 35 – 7. 45) (35 – 45) (80 – 110) (22 – 26) (> 90 %) Dr. Mazen Alzaharna CC 2018/1 26

• Based on the blood gas data drawn during the first admission to the emergency department (ED), the patient is not hypoxic. • The PO 2, SO 2, and Sp. O 2 are normal. • No further tests were run, and the patient recovered after several hours in the ED. Dr. Mazen Alzaharna CC 2018/1 27
• Considering the new data obtained from the cooximeter on second admission, the patient is hypoxic. • The O 2 Hb, determined spectrophotometrically, reflects the amount of O 2 actually bound to hemoglobin. The SO 2 is from a calculation based on the assumption that only normal hemoglobin is present. Sp. O 2 assessment of oxygen saturation from a pulse oximeter also assumes that only normal hemoglobin is present. O 2 Hb is an actual measurement that considers all hemoglobin species and reflects the amount of oxygen actually bound to hemoglobin. Dr. Mazen Alzaharna CC 2018/1 28
• The presence of carbon monoxide (CO) is reflected in the elevated COHb value. • This patient was seen in the fall, shortly after turning on the furnace in his home. • He was suffering from CO poisoning. Dr. Mazen Alzaharna CC 2018/1 29
- Slides: 29