Blepharospasm A Rare Focal Dystonia and the Role

Blepharospasm: A Rare, Focal Dystonia, and the Role of BOTOX® Treatment <Presenter Name> <Presenter Credentials> Please see Indication and Important Safety Information, including Boxed Warning, throughout this presentation.

2 IMPORTANT SAFETY INFORMATION, INCLUDING BOXED WARNING: DISTANT SPREAD OF TOXIN EFFECT Postmarketing reports indicate that the effects of BOTOX® and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These may include asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence, and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening, and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity, but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have an underlying condition that would predispose them to these symptoms. In unapproved uses, including spasticity in children, and in approved indications, cases of spread of effect have been reported at doses comparable to those used to treat cervical dystonia and spasticity and at lower doses. Please see Indication and additional Important Safety Information throughout this presentation.
3 What Type of Patients Will We Be Discussing Today? Indication Blepharospasm BOTOX® for injection is indicated for the treatment of blepharospasm associated with dystonia, including benign essential blepharospasm or VII nerve disorders in patients 12 years of age and above. Please see Important Safety Information, including Boxed Warning, throughout this presentation.

4 Agenda • Overview of blepharospasm and clinical guideline for diagnosis • Clinical trial profile for BOTOX® in blepharospasm Please see Important Safety Information, including Boxed Warning, throughout this presentation.
5 Disease Overview, Diagnosis, and Treatment Please see Important Safety Information, including Boxed Warning, throughout this presentation.
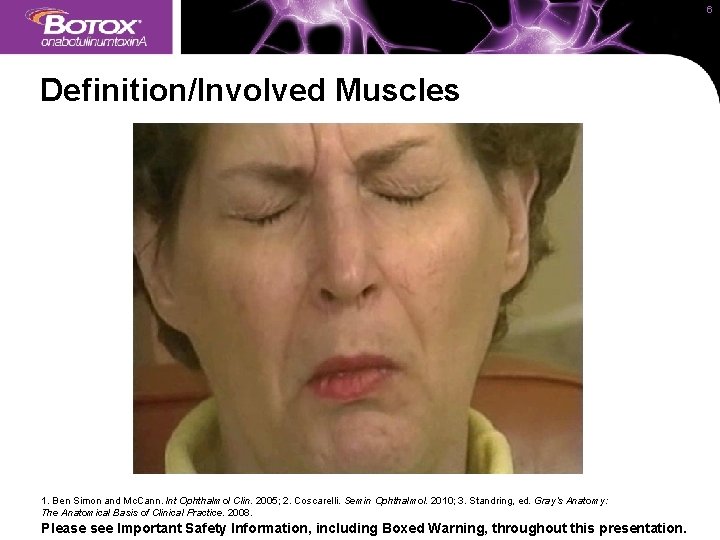
6 Definition/Involved Muscles • Blepharospasm is a focal dystonia characterized by sustained, involuntary spasms of eyelid closure 1 • Muscles affected are innervated by the temporal division of the facial nerve (VII)2: – The image below shows the functional anatomy of muscles involved in blepharospasm. Muscles for anatomical reference only are noted as such Procerus* Pulls the inner angle of the eyebrows downward and produces transverse nasal wrinkles 3 Orbicularis oculi Closes, blinks, and squints eyes 3 Corrugator supercilii* Pulls the eyebrows down toward the bridge of the nose resulting in “frowning” motion 3 *For anatomical reference only. Lines indicate general areas, not the specific injection sites. 1. Ben Simon and Mc. Cann. Int Ophthalmol Clin. 2005; 2. Coscarelli. Semin Ophthalmol. 2010; 3. Standring, ed. Gray’s Anatomy: The Anatomical Basis of Clinical Practice. 2008. Please see Important Safety Information, including Boxed Warning, throughout this presentation.

7 A Rare, Focal Dystonia • Epidemiological studies have determined blepharospasm is a rare condition – Estimates range from 235 to 22, 518 patients with blepharospasm in the United States 1, 2 • Onset usually occurs in patients between fifth and seventh decade of life 3 • Blepharospasm can impair many patient activities, such as driving, walking, reading, and watching TV 4 Due to its rarity, patients may go years before reaching a specialist who is able to diagnose them with blepharospasm 1. Nutt et al. Mov Disord. 1988; 2. CDC WONDER website. Census projections page. Accessed 2016; 3. Brin et al. In: Moore and Naumann, eds. Handbook of Botulinum Toxin Treatment. 2003; 4. Ben Simon and Mc. Cann. Int Ophthalmol Clin. 2005. Please see Important Safety Information, including Boxed Warning, throughout this presentation.

8 Signs and Symptoms • Early symptoms include 1, 2: – – – Dry eyes Light sensitivity Increased blinking Ocular pain Soreness • Frequent blinking is a progressive symptom 1 • Bright light, polluted air, and wind can worsen symptoms 1 Patients may self-manage these symptoms by wearing dark glasses and using eye drops 2 1. Coscarelli. Semin Ophthalmol. 2010; 2. Ben Simon and Mc. Cann. Int Ophthalmol Clin. 2005. Please see Important Safety Information, including Boxed Warning, throughout this presentation.
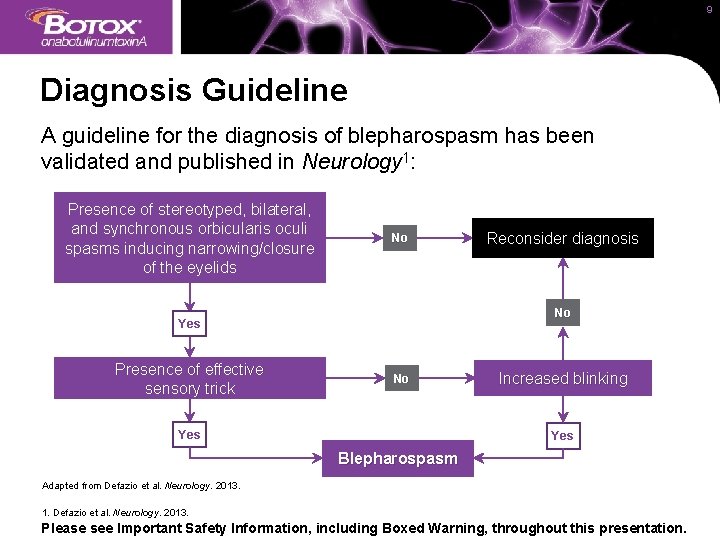
9 Diagnosis Guideline A guideline for the diagnosis of blepharospasm has been validated and published in Neurology 1: Presence of stereotyped, bilateral, and synchronous orbicularis oculi spasms inducing narrowing/closure of the eyelids No No Yes Presence of effective sensory trick Reconsider diagnosis No Yes Increased blinking Yes Blepharospasm Adapted from Defazio et al. Neurology. 2013. 1. Defazio et al. Neurology. 2013. Please see Important Safety Information, including Boxed Warning, throughout this presentation.
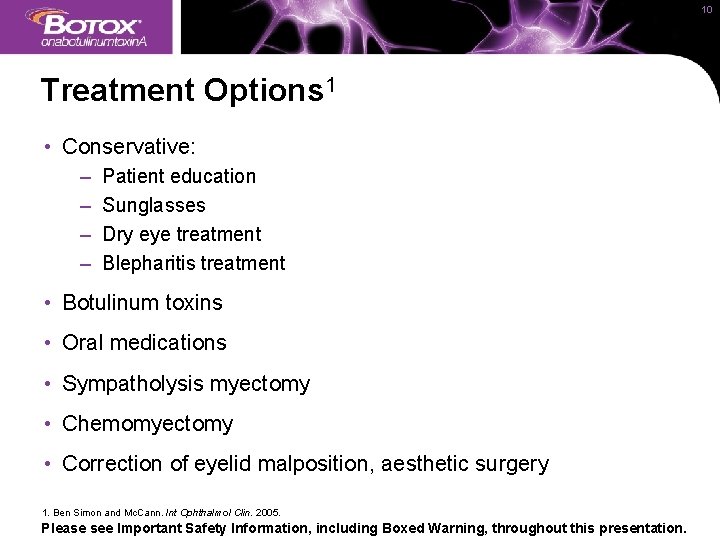
10 Treatment Options 1 • Conservative: – – Patient education Sunglasses Dry eye treatment Blepharitis treatment • Botulinum toxins • Oral medications • Sympatholysis myectomy • Chemomyectomy • Correction of eyelid malposition, aesthetic surgery 1. Ben Simon and Mc. Cann. Int Ophthalmol Clin. 2005. Please see Important Safety Information, including Boxed Warning, throughout this presentation.

11 BOTOX® Was the First Therapy Approved for the Treatment of Blepharospasm More Than 25 Years Ago • An ophthalmologist, Dr. Alan Scott, began experimenting with therapeutic use of botulinum toxin in the 1960 s 1 • In 1968, he began collaborating with Dr. Edward Schantz and began human testing of BOTOX® in the 1970 s after years of animal testing 1 • BOTOX® was approved by the FDA for the treatment of blepharospasm in 19892 – Prior to the introduction of BOTOX® no treatment had been approved by the FDA for the treatment of blepharospasm 3 1. Erbguth. J Neural Transm. 2008; 2. BOTOX® Prescribing Information, January 2016; 3. BEBRF website. Blepharospasm pages. Accessed 2016. Please see Important Safety Information, including Boxed Warning, throughout this presentation.

12 BOTOX® Clinical Profile Please see Important Safety Information, including Boxed Warning, throughout this presentation.
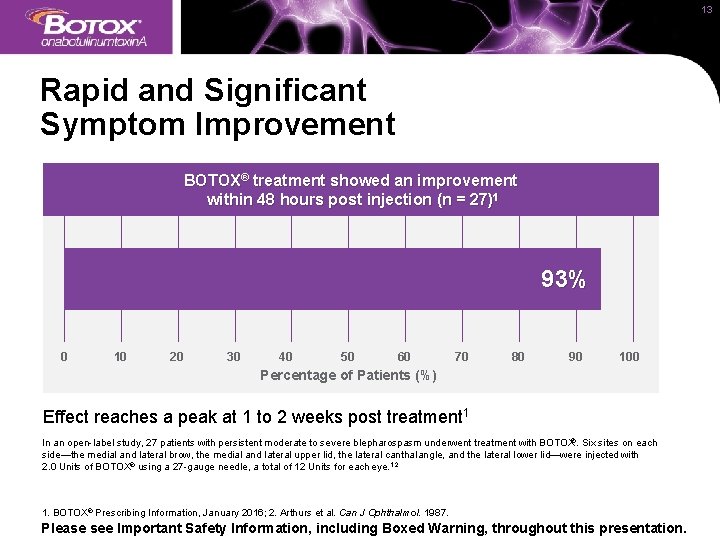
13 Rapid and Significant Symptom Improvement BOTOX® treatment showed an improvement within 48 hours post injection (n = 27)1 93% 0 10 20 30 40 50 60 70 80 90 100 Percentage of Patients (%) Effect reaches a peak at 1 to 2 weeks post treatment 1 In an open-label study, 27 patients with persistent moderate to severe blepharospasm underwent treatment with BOTOX®. Six sites on each side—the medial and lateral brow, the medial and lateral upper lid, the lateral canthal angle, and the lateral lower lid—were injected with 2. 0 Units of BOTOX® using a 27 -gauge needle, a total of 12 Units for each eye. 1, 2 1. BOTOX® Prescribing Information, January 2016; 2. Arthurs et al. Can J Ophthalmol. 1987. Please see Important Safety Information, including Boxed Warning, throughout this presentation.
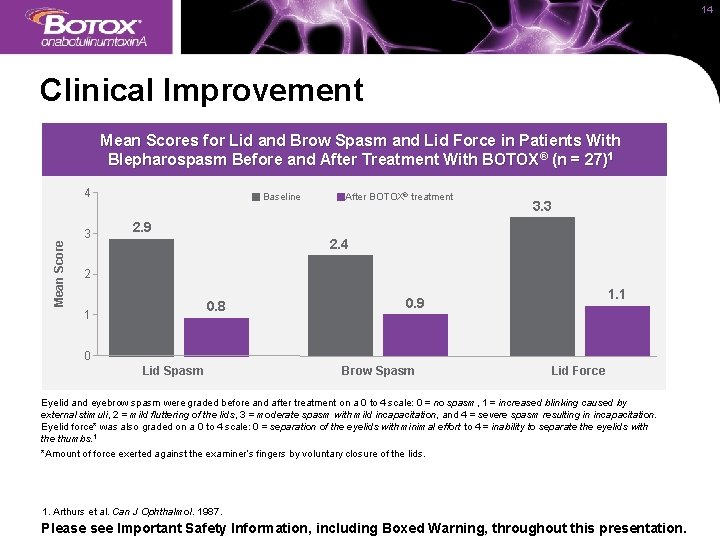
14 Clinical Improvement Mean Scores for Lid and Brow Spasm and Lid Force in Patients With Blepharospasm Before and After Treatment With BOTOX® (n = 27)1 Mean Score 4 3 Baseline After BOTOX® treatment 3. 3 2. 9 2. 4 2 0. 8 1 1. 1 0. 9 0 Lid Spasm Brow Spasm Lid Force Eyelid and eyebrow spasm were graded before and after treatment on a 0 to 4 scale: 0 = no spasm, 1 = increased blinking caused by external stimuli, 2 = mild fluttering of the lids, 3 = moderate spasm with mild incapacitation, and 4 = severe spasm resulting in incapacitation. Eyelid force* was also graded on a 0 to 4 scale: 0 = separation of the eyelids with minimal effort to 4 = inability to separate the eyelids with the thumbs. 1 *Amount of force exerted against the examiner’s fingers by voluntary closure of the lids. 1. Arthurs et al. Can J Ophthalmol. 1987. Please see Important Safety Information, including Boxed Warning, throughout this presentation.
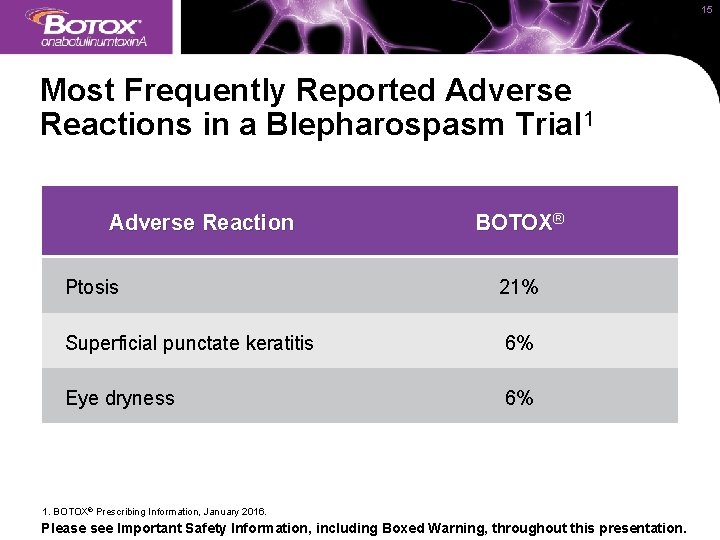
15 Most Frequently Reported Adverse Reactions in a Blepharospasm Trial 1 Adverse Reaction BOTOX® Ptosis 21% Superficial punctate keratitis 6% Eye dryness 6% 1. BOTOX® Prescribing Information, January 2016. Please see Important Safety Information, including Boxed Warning, throughout this presentation.
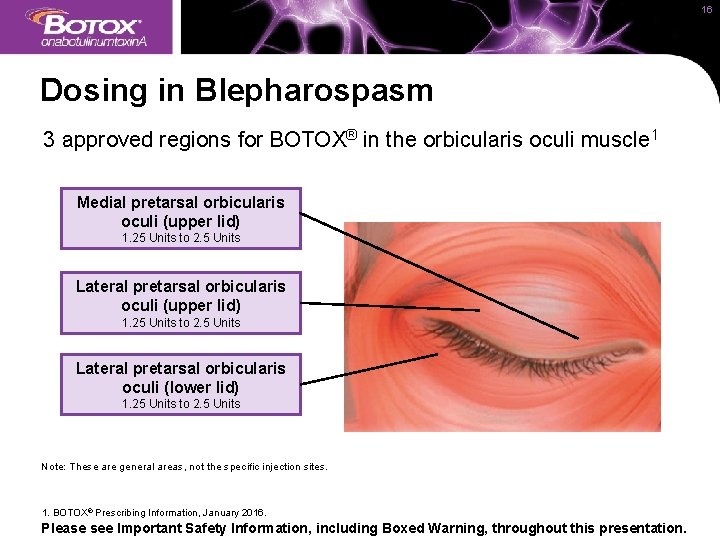
16 Dosing in Blepharospasm 3 approved regions for BOTOX® in the orbicularis oculi muscle 1 Medial pretarsal orbicularis oculi (upper lid) 1. 25 Units to 2. 5 Units Lateral pretarsal orbicularis oculi (lower lid) 1. 25 Units to 2. 5 Units Note: These are general areas, not the specific injection sites. 1. BOTOX® Prescribing Information, January 2016. Please see Important Safety Information, including Boxed Warning, throughout this presentation.

17 Dosing Units Are Not Interchangeable With Other Botulinum Toxin Products The potency Units of BOTOX® are specific to the preparation and assay method utilized and are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of BOTOX® cannot be compared to nor converted into units of any other botulinum toxin products assessed with any other specific assay method 1 1. BOTOX® Prescribing Information, January 2016. Please see Important Safety Information, including Boxed Warning, throughout this presentation.

18 Blepharospasm Dosing Considerations • The initial recommended dose is 1. 25 Units to 2. 5 Units at each site. Volume of injection: 0. 05 m. L to 0. 1 m. L per site 1 • The cumulative dose of BOTOX® treatment for blepharospasm in a 30 -day period should not exceed 200 Units 1 • Reconstituted BOTOX® is injected using a sterile, 27 - to 30 -gauge needle without electromyographic guidance 1 • Avoiding injection near the levator palpebrae superioris may reduce the complication of ptosis 1 • Avoiding medial lower lid injections, and thereby reducing diffusion into the inferior oblique, may reduce the complication of diplopia. Ecchymosis can be prevented by applying pressure at the injection site immediately after injection 1 • Initial effect of the injections is generally seen within 3 days and reaches a peak 1 to 2 weeks post treatment. Each treatment lasts approximately 3 months, following which the procedure can be repeated 1 1. BOTOX® Prescribing Information, January 2016. Please see Important Safety Information, including Boxed Warning, throughout this presentation.

19 General Dosing Considerations • Indication-specific dosage and administration recommendations should be followed. When initiating treatment, the lowest recommended dose should be used. In treating adult patients for 1 or more indications, the maximum cumulative dose should not exceed 400 Units in a 3 -month interval 1 • The safe and effective use of BOTOX® depends upon proper storage of the product, selection of the correct dose, and proper reconstitution and administration techniques. Physicians administering BOTOX® must understand the relevant neuromuscular and structural anatomy of the area involved any alterations to the anatomy due to prior surgical procedures and disease, especially when injecting near the lungs 1 1. BOTOX® Prescribing Information, January 2016. Please see Important Safety Information, including Boxed Warning, throughout this presentation.

20 IMPORTANT SAFETY INFORMATION (continued) CONTRAINDICATIONS BOTOX® is contraindicated in the presence of infection at the proposed injection site(s) and in individuals with known hypersensitivity to any botulinum toxin preparation or to any of the components in the formulation. WARNINGS AND PRECAUTIONS Lack of Interchangeability Between Botulinum Toxin Products The potency Units of BOTOX® are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of BOTOX ® cannot be compared to nor converted into units of any other botulinum toxin products assessed with any other specific assay method. Spread of Toxin Effect See Boxed Warning. No definitive serious adverse event reports of distant spread of toxin effect associated with BOTOX® for blepharospasm at the recommended dose (30 Units and below) have been reported.

21 IMPORTANT SAFETY INFORMATION (continued) WARNINGS AND PRECAUTIONS (continued) Serious Adverse Reactions With Unapproved Use Serious adverse reactions, including excessive weakness, dysphagia, and aspiration pneumonia, with some adverse reactions associated with fatal outcomes, have been reported in patients who received BOTOX® injections for unapproved uses. In these cases, the adverse reactions were not necessarily related to distant spread of toxin, but may have resulted from the administration of BOTOX® to the site of injection and/or adjacent structures. In several of the cases, patients had pre-existing dysphagia or other significant disabilities. There is insufficient information to identify factors associated with an increased risk for adverse reactions associated with the unapproved uses of BOTOX®. The safety and effectiveness of BOTOX® for unapproved uses have not been established. Hypersensitivity Reactions Serious and/or immediate hypersensitivity reactions have been reported. These reactions include anaphylaxis, serum sickness, urticaria, soft-tissue edema, and dyspnea. If such a reaction occurs, further injection of BOTOX® should be discontinued and appropriate medical therapy immediately instituted. One fatal case of anaphylaxis has been reported in which lidocaine was used as the diluent, and consequently the causal agent cannot be reliably determined.

22 IMPORTANT SAFETY INFORMATION (continued) WARNINGS AND PRECAUTIONS (continued) Increased Risk of Clinically Significant Effects With Pre-Existing Neuromuscular Disorders Individuals with peripheral motor neuropathic diseases, amyotrophic lateral sclerosis, or neuromuscular junction disorders (eg, myasthenia gravis or Lambert-Eaton syndrome) should be monitored when given botulinum toxin. Patients with neuromuscular disorders may be at increased risk of clinically significant effects including generalized muscle weakness, diplopia, ptosis, dysphonia, dysarthria, severe dysphagia, and respiratory compromise from therapeutic doses of BOTOX® (see Warnings and Precautions). Dysphagia and Breathing Difficulties Treatment with BOTOX® and other botulinum toxin products can result in swallowing or breathing difficulties. Patients with pre-existing swallowing or breathing difficulties may be more susceptible to these complications. In most cases, this is a consequence of weakening of muscles in the area of injection that are involved in breathing or oropharyngeal muscles that control swallowing or breathing (see Boxed Warning).

23 IMPORTANT SAFETY INFORMATION (continued) WARNINGS AND PRECAUTIONS (continued) Corneal Exposure and Ulceration in Patients Treated With BOTOX® for Blepharospasm Reduced blinking from BOTOX® injection of the orbicularis muscle can lead to corneal exposure, persistent epithelial defect, and corneal ulceration, especially in patients with VII nerve disorders. Human Albumin and Transmission of Viral Diseases This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD) is also considered extremely remote. No cases of transmission of viral diseases or CJD have ever been reported for albumin.

24 IMPORTANT SAFETY INFORMATION (continued) ADVERSE REACTIONS The following adverse reactions to BOTOX® for injection are discussed in greater detail in the following sections: Spread of Toxin Effect (see Boxed Warning); Serious Adverse Reactions With Unapproved Use (see Warnings and Precautions); Hypersensitivity Reactions (see Contraindications and Warnings and Precautions); Increased Risk of Clinically Significant Effects With Pre-Existing Neuromuscular Disorders (see Warnings and Precautions); Dysphagia and Breathing Difficulties (see Warnings and Precautions); and Corneal Exposure and Ulceration in Patients Treated for Blepharospasm (see Warnings and Precautions). Blepharospasm The most frequently reported adverse reactions following injection of BOTOX® for blepharospasm include ptosis (21%), superficial punctate keratitis (6%), and eye dryness (6%).

25 IMPORTANT SAFETY INFORMATION (continued) ADVERSE REACTIONS (continued) Post Marketing Experience There have been spontaneous reports of death, sometimes associated with dysphagia, pneumonia, and/or other significant debility or anaphylaxis, after treatment with botulinum toxin. There have also been reports of adverse events involving the cardiovascular system, including arrhythmia and myocardial infarction, some with fatal outcomes. Some of these patients had risk factors including cardiovascular disease. The exact relationship of these events to the botulinum toxin injection has not been established. DRUG INTERACTIONS Co-administration of BOTOX® and aminoglycosides or other agents interfering with neuromuscular transmission (eg, curare-like compounds) should only be performed with caution as the effect of the toxin may be potentiated. Use of anticholinergic drugs after administration of BOTOX® may potentiate systemic anticholinergic effects. The effect of administering different botulinum neurotoxin products at the same time or within several months of each other is unknown. Excessive neuromuscular weakness may be exacerbated by administration of another botulinum toxin prior to the resolution of the effects of a previously administered botulinum toxin. Excessive weakness may also be exaggerated by administration of a muscle relaxant before or after administration of BOTOX®. Please see full Prescribing Information including Boxed Warning and Medication Guide.

26 Summary • Blepharospasm is a focal dystonia characterized by sustained, involuntary spasms of eyelid closure • A clinical guideline for the diagnosis of blepharospasm has been validated and published in Neurology • In 1989, BOTOX® became the first therapy approved by the FDA for the treatment of blepharospasm • BOTOX® provides rapid and significant symptom improvement Please see Important Safety Information, including Boxed Warning, throughout this presentation.

Thank You! © 2016 Allergan. All rights reserved. All trademarks are the property of their respective owners. BOTOXMedical. com APC 67 TU 16 160933
- Slides: 27