BLACKBODY RADIATION PLANCKS LAW COLOR and SPECTRAL CLASS


BLACKBODY RADIATION: PLANCK’S LAW

COLOR and SPECTRAL CLASS • The light emitted by stars consists of a mixture of all colors, but our eyes (and brain) perceive such light as being white or tinged with pastel color. • In fact, different stars have varying amounts of each color in their light; this causes stars to have different colors. • Most people, however, have never noticed that stars come in a variety of colors. • When light from the Sun (or any other star) is passed through a prism, it is separated into its component colors -- a continuous spectrum.
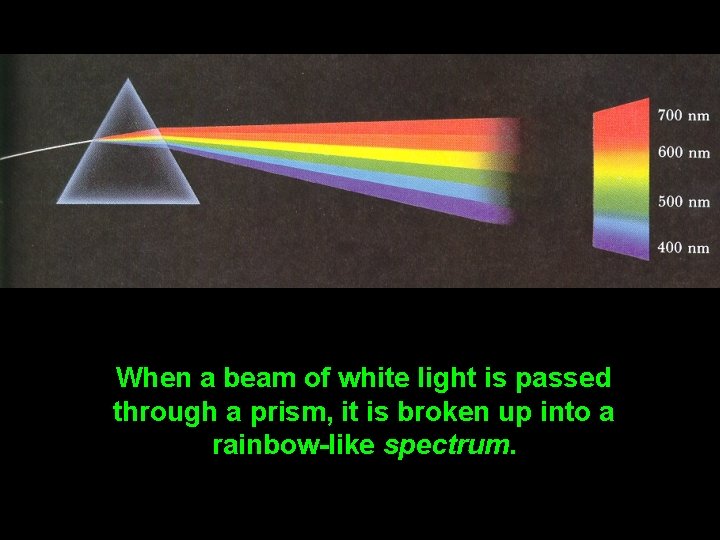
When a beam of white light is passed through a prism, it is broken up into a rainbow-like spectrum.

COLOR and SPECTRAL CLASS • If the spectra of different stars are analyzed, it is found that the intensity of the various colors differs from star to star. • Relatively cool stars have their peak intensity in the red or orange part of the spectrum. • The hottest stars emit blue light most strongly. • In other words, the color (or wavelength, ) of the maximum intensity depends upon the temperature of the star. • The star is not necessarily the color of the maximum intensity; in fact, there are no green stars.
Max Karl Ernst Ludwig Planck 1858 - 1947

Max Planck 1858 - 1947 • In the late 1890’s, Wien and Rayleigh had unsuccessfully attempted to formulate an equation expressing the intensity of electromagnetic radiation as a function of wavelength and the temperature of the source. • In 1900, Planck derived the equation empirically. • By December of 1900, Planck had derived the equation from fundamental principles.
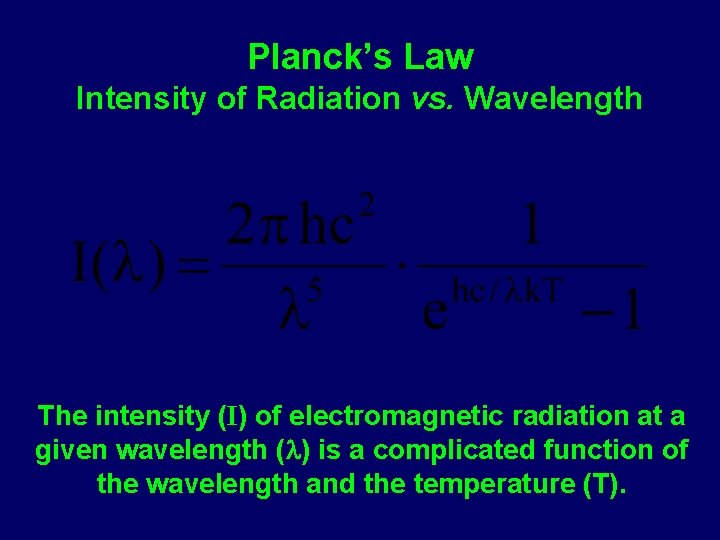
Planck’s Law Intensity of Radiation vs. Wavelength The intensity (I) of electromagnetic radiation at a given wavelength ( ) is a complicated function of the wavelength and the temperature (T).
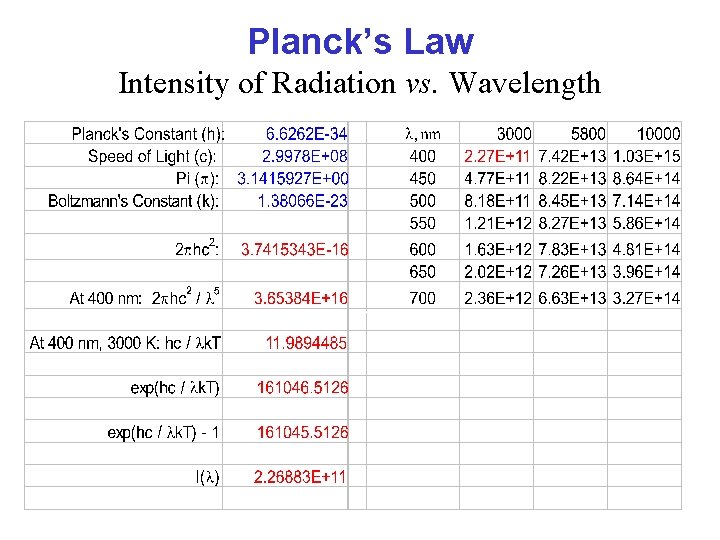
Planck’s Law Intensity of Radiation vs. Wavelength
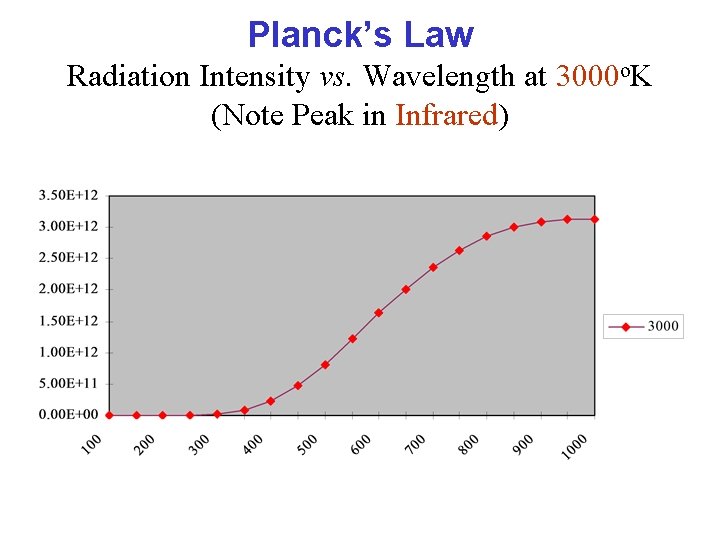
Planck’s Law Radiation Intensity vs. Wavelength at 3000 o. K (Note Peak in Infrared)
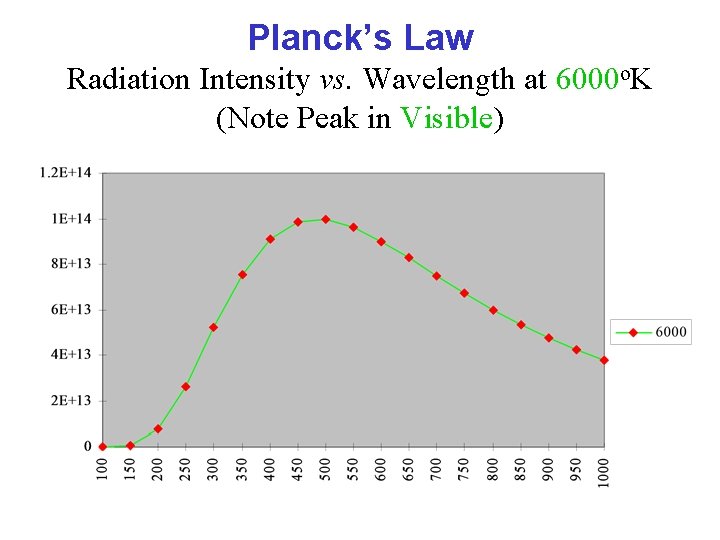
Planck’s Law Radiation Intensity vs. Wavelength at 6000 o. K (Note Peak in Visible)
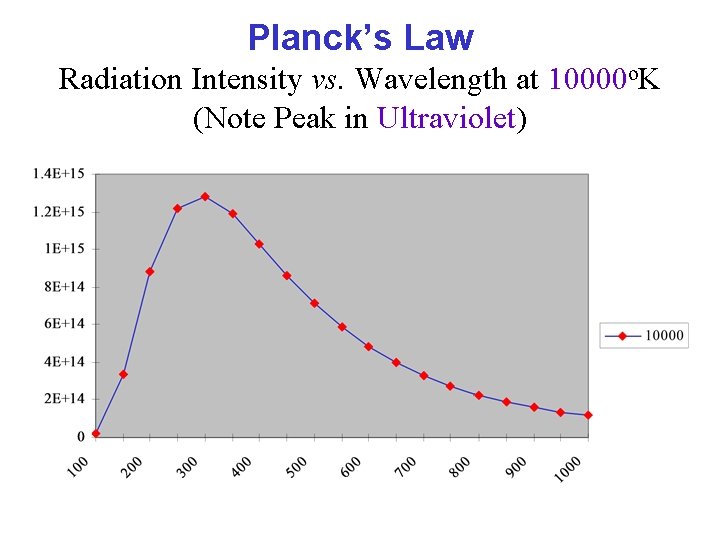
Planck’s Law Radiation Intensity vs. Wavelength at 10000 o. K (Note Peak in Ultraviolet)
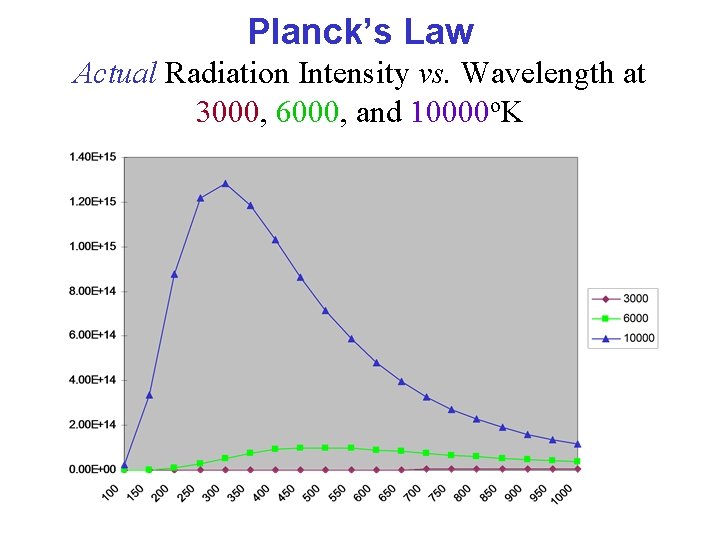
Planck’s Law Actual Radiation Intensity vs. Wavelength at 3000, 6000, and 10000 o. K
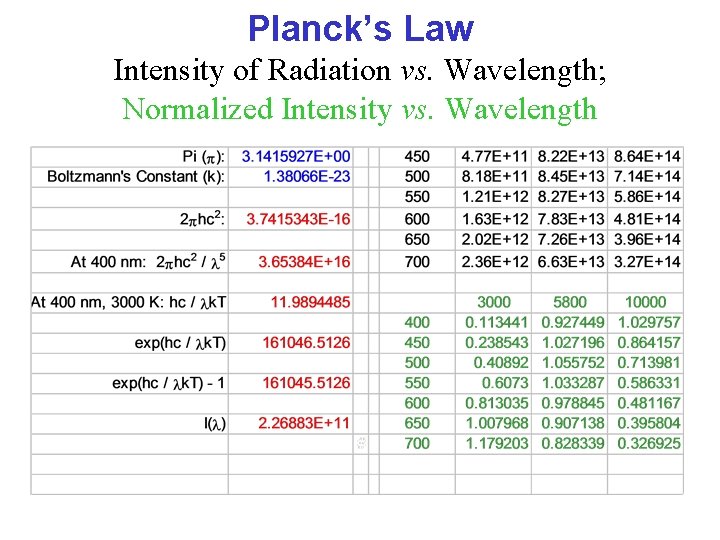
Planck’s Law Intensity of Radiation vs. Wavelength; Normalized Intensity vs. Wavelength
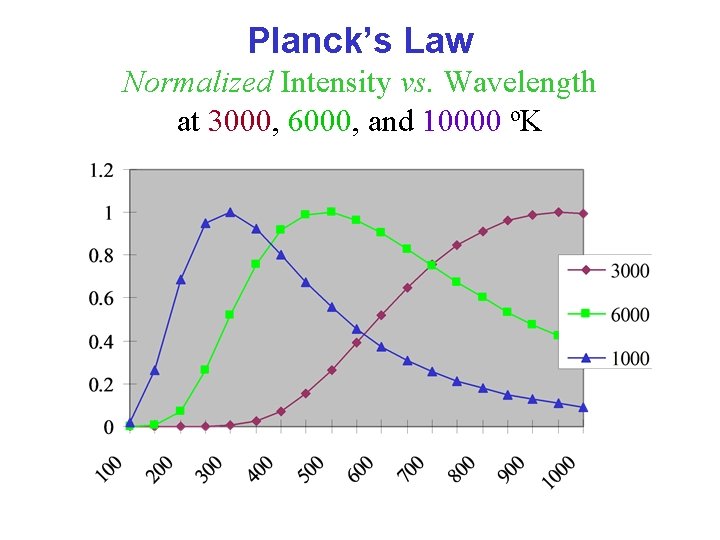
Planck’s Law Normalized Intensity vs. Wavelength at 3000, 6000, and 10000 o. K
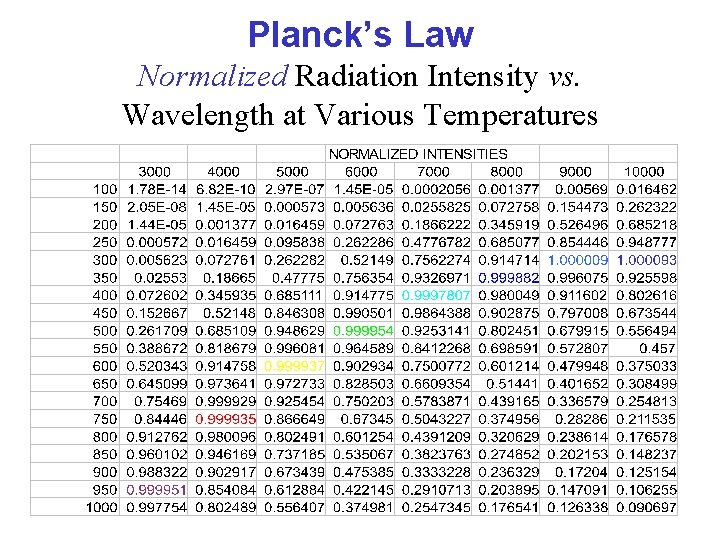
Planck’s Law Normalized Radiation Intensity vs. Wavelength at Various Temperatures
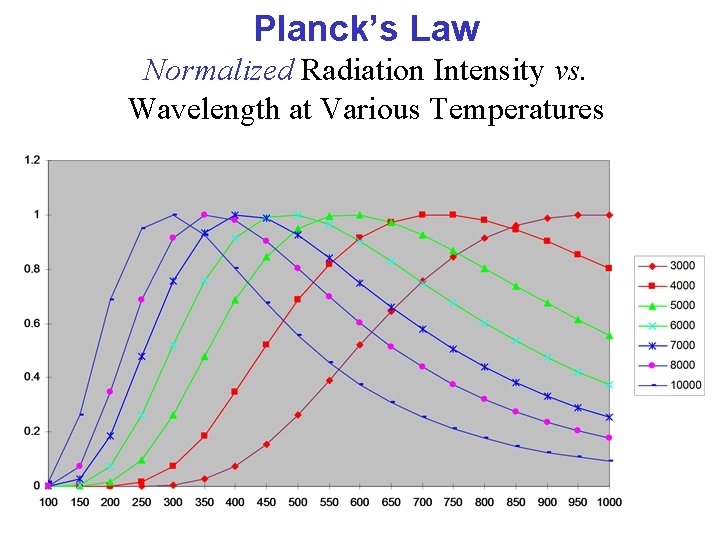
Planck’s Law Normalized Radiation Intensity vs. Wavelength at Various Temperatures
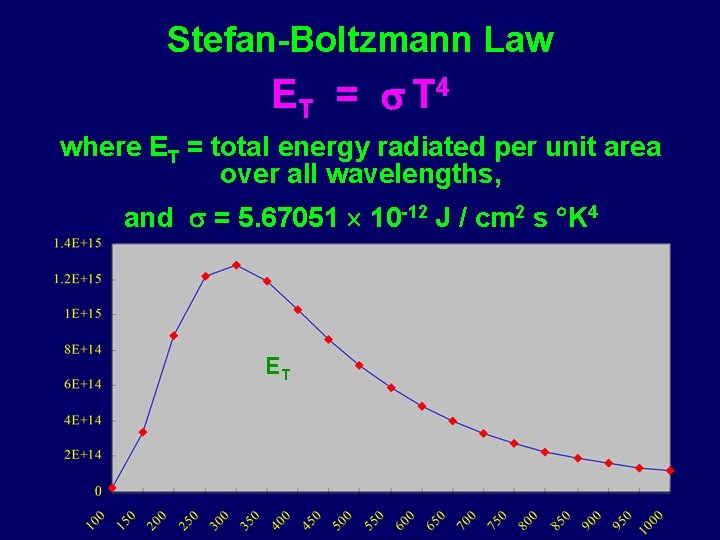
Stefan-Boltzmann Law ET = T 4 where ET = total energy radiated per unit area over all wavelengths, and = 5. 67051 10 -12 J / cm 2 s K 4 ET

Wilhelm Carl Werner Otto Fritz Franz Wien 1864 - 1928

Wilhelm Wien 1864 - 1928 • In 1896, Wilhelm Wien unsuccessfully attempted to derive what is now known as Planck’s Law. • However, he did notice a relationship between the temperature of a glowing object and the wavelength of its maximum intensity of emission. • The result of his investigation is now known as Wien’s Displacement Law.
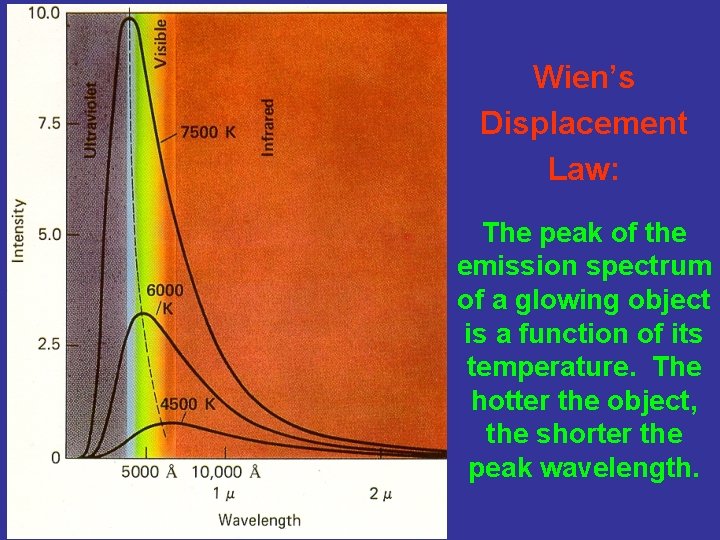
Wien’s Displacement Law: The peak of the emission spectrum of a glowing object is a function of its temperature. The hotter the object, the shorter the peak wavelength.

Wien’s Displacement Law Gives max as f(T), which allows us to calculate the temperature of a star if we know the wavelength of its maximum emission, which is easy to measure from its spectrum. From Planck’s Law, take d. I/d , set = 0. Then, max T = 2. 8979 106 nm K. Example: max for the Sun = 502 nm. Therefore, T = 5770 K = 5500 C.
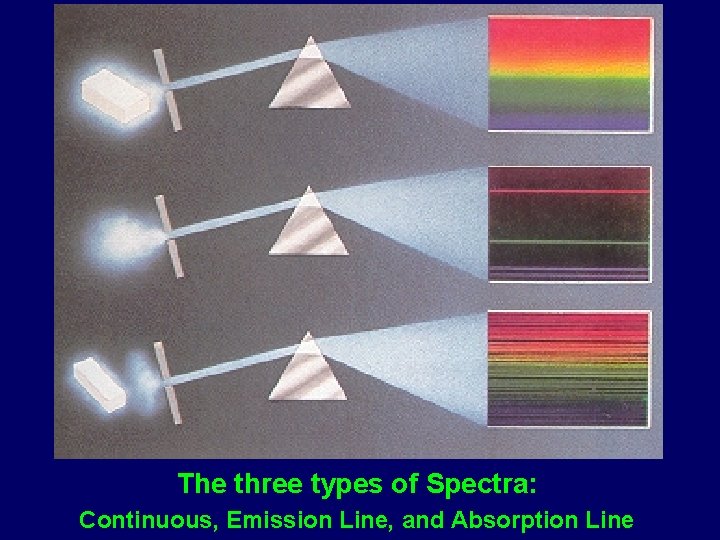
The three types of Spectra: Continuous, Emission Line, and Absorption Line
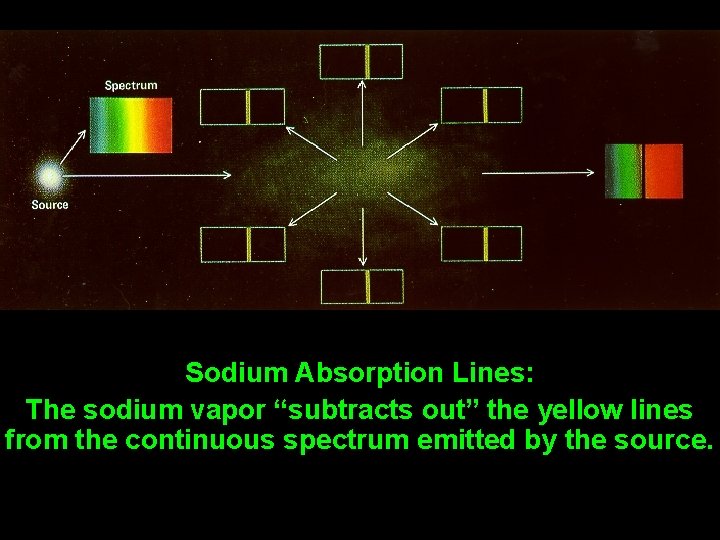
Sodium Absorption Lines: The sodium vapor “subtracts out” the yellow lines from the continuous spectrum emitted by the source.
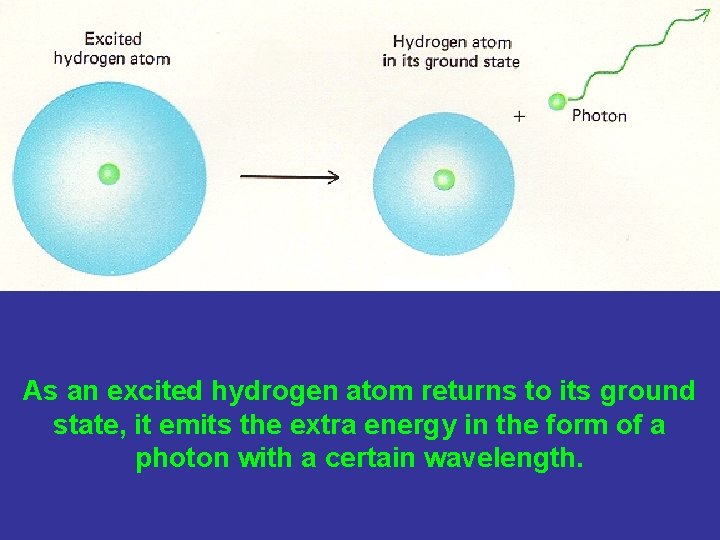
As an excited hydrogen atom returns to its ground state, it emits the extra energy in the form of a photon with a certain wavelength.
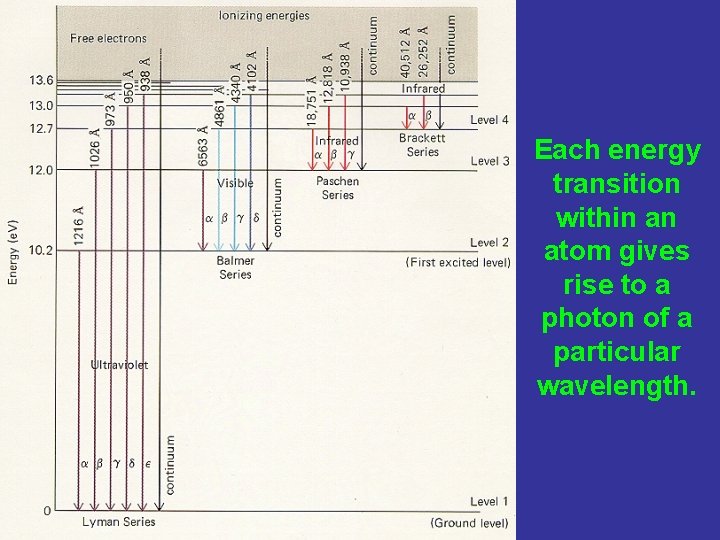
Each energy transition within an atom gives rise to a photon of a particular wavelength.
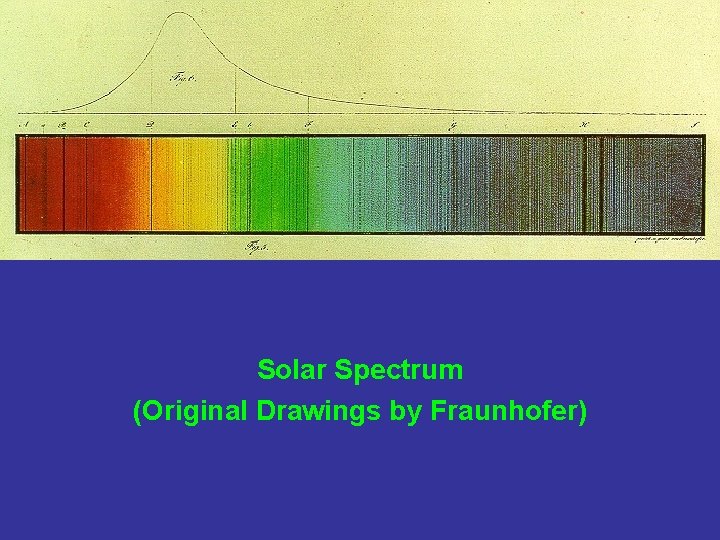
Solar Spectrum (Original Drawings by Fraunhofer)
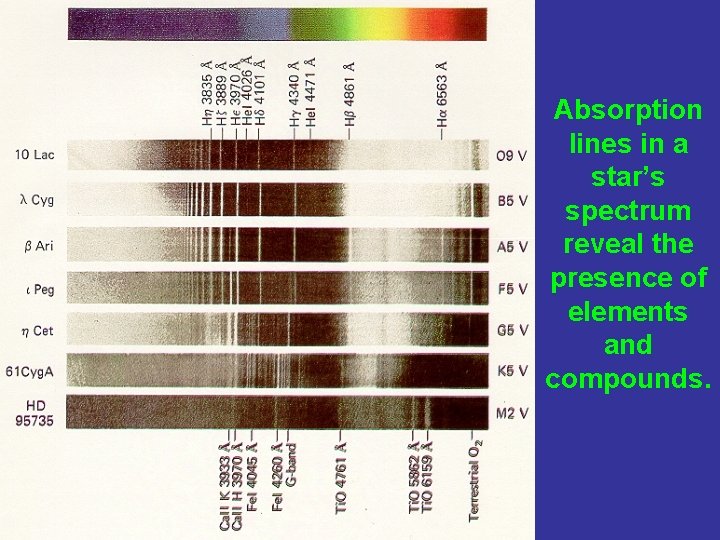
Absorption lines in a star’s spectrum reveal the presence of elements and compounds.
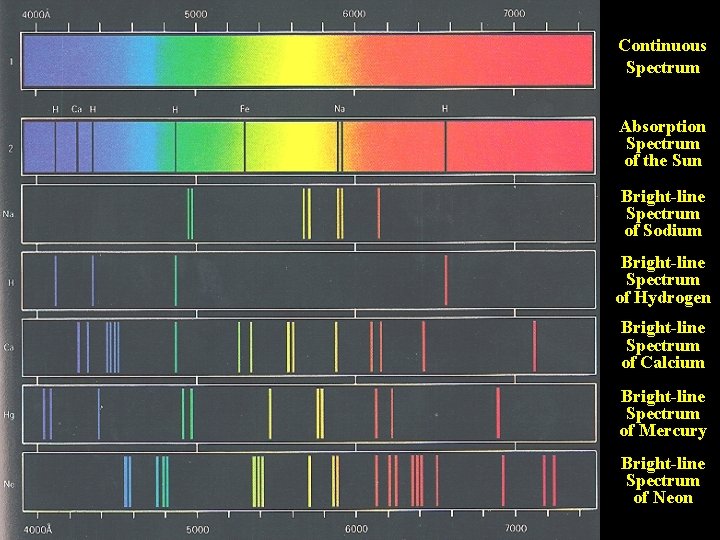
Continuous Spectrum Absorption Spectrum of the Sun Bright-line Spectrum of Sodium Bright-line Spectrum of Hydrogen Bright-line Spectrum of Calcium Bright-line Spectrum of Mercury Bright-line Spectrum of Neon
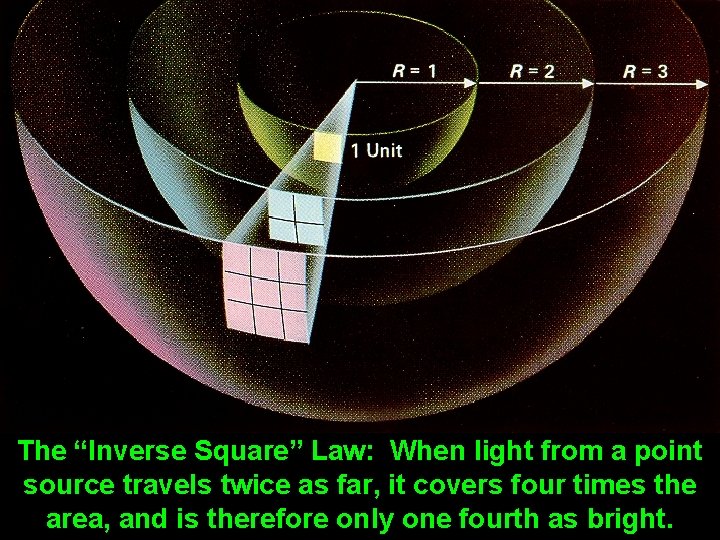
The “Inverse Square” Law: When light from a point source travels twice as far, it covers four times the area, and is therefore only one fourth as bright.

THE END

- Slides: 32