BIURET TEST For protein detection and quantitative analysis
BIURET TEST For protein detection and quantitative analysis

Aim of Biuret Test ◦ The Biuret Test is done to show the presence of peptide bonds, which are the basis for the formation of proteins. These bonds will make the blue Biuret reagent turn purple.
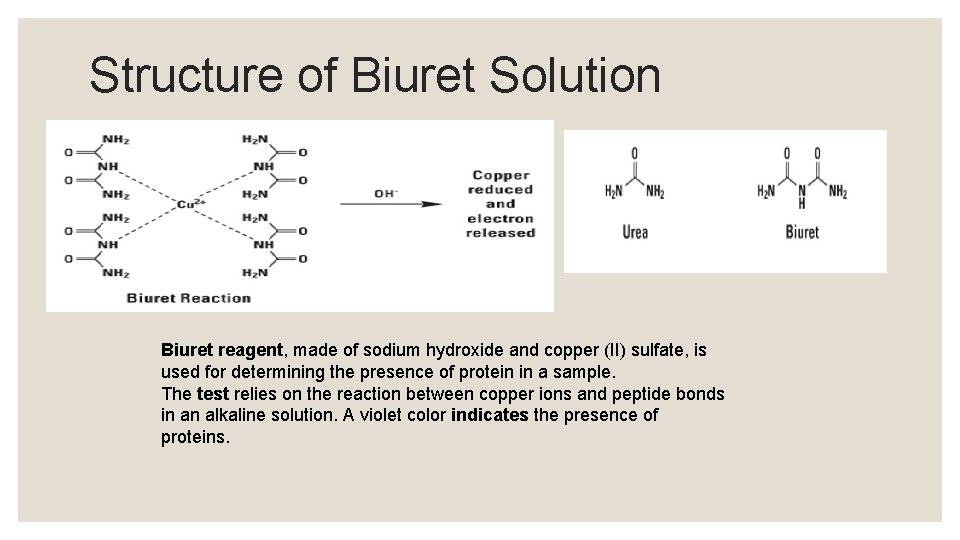
Structure of Biuret Solution Biuret reagent, made of sodium hydroxide and copper (II) sulfate, is used for determining the presence of protein in a sample. The test relies on the reaction between copper ions and peptide bonds in an alkaline solution. A violet color indicates the presence of proteins.

◦ Peptides contain three or more amino acid residues form a colored chelate complex with cupric ions (Cu 2+) in an alkaline enviroment contain sodium potassium tartrate. ◦ Single amino acids and dipeptide do not react with biuret solution but tripeptides and larger polypeptides or proteins will react to produce the blue to violet complex that absorbs the light at 540 nm. ◦ One cupric ion forms a colored coordination complex with four to six nearby peptides bonds. Color intensity produced is proportional to th number of peptide bonds present in the reaction.

Principle • The biuret reagent (copper sulfate in a strong base) reacts with peptide bonds in proteins to form a blue to violet complex known as the “biuret complex”. • N. B. Two peptide bonds at least are required for the formation of this complex.

Procedure ◦ Sample Preparation ◦ Measure different amount of Egg Albumin (0. 250, 0. 500, 0. 750, 1. 000)g individually. ◦ Use distilled water for preparing clear solution of egg albumin (50 ml) of each. ◦ Stir it well with glass rod. ◦ Measure 0. 500 g of Glycine on Sensitive balance. ◦ Use distilled water for preparing clear solution of Glycine solution (100 ml). ◦ Stir it well with glass rod. ◦ Biuret Reagent Preparation ◦ Measure 8 g of Na. OH on sensitive balance. ◦ Use distilled water for preparing clear solution of Na. OH Solution (100 ml). ◦ Stir it well with glass rod. ◦ Measure 1 g of Cu. SO 4 on Sensitive balance. ◦ Use distilled water for preparing clear solution of Cu. SO 4 Solution (100 ml). ◦ Stir it well with glass rod.

Observations Interpretation No change ( solution remains blue) Proteins are not present The solution turns from blue to violet Proteins are present The solution turns from blue to pink Peptides or peptones are short chains of amino acid residues
- Slides: 7