BIOSTATISTICS Contents 1 Clinical Trial Science or Ethics


BIOSTATISTICS Contents 1. Clinical Trial – Science or Ethics? 2. Clinical Questions & Outcomes 3. Statistical Principles − Controls / Randomization / Blinding 4. Types of Clinical Trials 5. Statistical Analyses 6. Data Set 7. Data Management 8. Sample size Calculation 9. Roles of Biostatistics 10. Conclusion 2/65
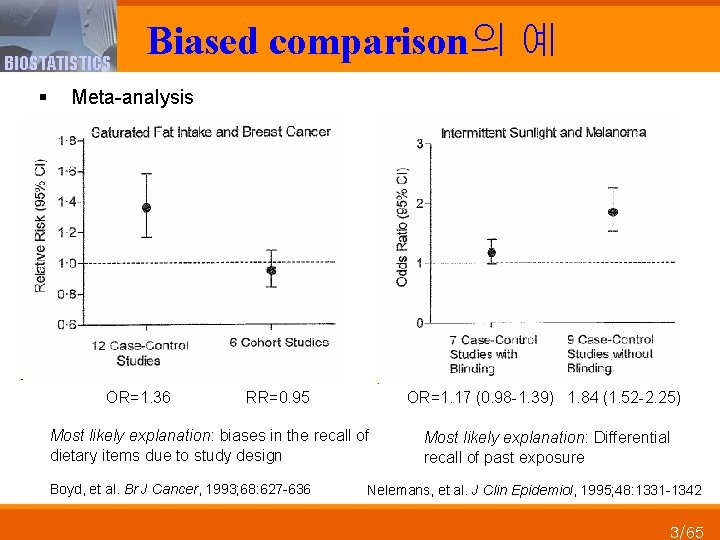
BIOSTATISTICS Biased comparison의 예 § Meta-analysis OR=1. 36 RR=0. 95 OR=1. 17 (0. 98 -1. 39) 1. 84 (1. 52 -2. 25) Most likely explanation: biases in the recall of dietary items due to study design Most likely explanation: Differential recall of past exposure Boyd, et al. Br J Cancer, 1993; 68: 627 -636 Nelemans, et al. J Clin Epidemiol, 1995; 48: 1331 -1342 3/65
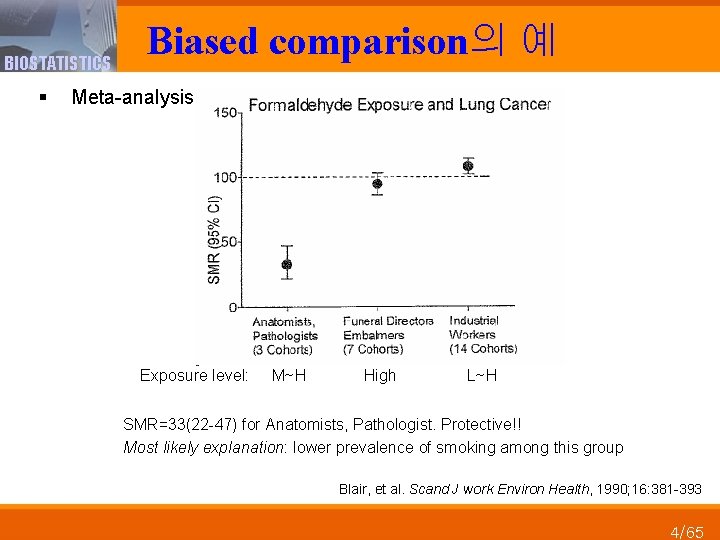
BIOSTATISTICS Biased comparison의 예 § Meta-analysis Exposure level: M~H High L~H SMR=33(22 -47) for Anatomists, Pathologist. Protective!! Most likely explanation: lower prevalence of smoking among this group Blair, et al. Scand J work Environ Health, 1990; 16: 381 -393 4/65

BIOSTATISTICS Clinical Trial – Scientific is ethical 5/65

BIOSTATISTICS Clinical research and clinical trials § Clinical research • A clinical study to evaluate clinical usefulness – Need both pathophysiologic and pharmacological insight – To verify a theory or hypothesis about efficacy of a medical intervention • Randomized Clinical Trial has become the paradigm – A means for measuring expected therapeutic-related benefits • Objective and scientific data의 생성 – From human subjects who receives an intervention of interest • Objectivity – Reproducibility of the results (재현성) – Repeatability of the results (반복성) – Transparency of the process (투명성) 6/65

Institutional Review Boards(IRB) BIOSTATISTICS § Required for each research institution § Must review each new protocol for • Merit and ethics • Informed consent / document § May provide limited scientific review • Design • Population studied • Adequacy of sample size § Must review protocol progress annually § Responsible for monitoring patient safety 7/65

BIOSTATISTICS Clinical Questions & Outcomes 8/65

BIOSTATISTICS Clinical outcome § Outcome • A (untoward) clinical event, over a fixed period of time • Occurrence would be verified for each patient • Characteristics 1. 2. 3. 4. 5. Well defined & stable Ascertained in all subjects Unbiased (objective) Reproducible Specificity to question 9/65
BIOSTATISTICS Primary vs. Secondary Question § Primary • • Most important, central question Ideally, only one Stated in advance Basis for design and sample size § Secondary • Related to primary • Stated in advance • Limited in number 10/65

BIOSTATISTICS Statistical Principles 11/65

BIOSTATISTICS Objectivity of clinical trial § Accuracy의 보장 • Lack of systematic error • Avoid bias • Not by proper analysis but by proper designing • 예: Controls, blinding, random process § Precision의 보장 • Lack of random error • Based on the size of the treatment groups • By an appropriate statistical model and analysis § Transparency의 보장 • The integrity of clinical trial data • By a qualified data management (DM) § Participants’ right and well-being의 보호 • Ethics 12/65

BIOSTATISTICS Systematic errors (Bias) § Systematic error에 영향을 미치는 요인들 • Extraneous factors (외생요인) – Attention of the physician – Psychology of patient – Adjustment life style – Co-medication • Prognostic factors (예후요인) – Risk profile of the patients • Information on outcome (결과에 관한 사전 정보) 13/65

BIOSTATISTICS Three Comparability Criteria § Treatment comparison이 관심 therapeutic effect에 관한 실제 차이를 반영하고 있는가? • Comparability of extraneous effects § Group들 간 prognosis는 비교 가능한가? • Comparability of prognosis § Group들 간 outcome은 동일한 선 상에서 관찰 되고 있는가? • Comparability of information 14/65

BIOSTATISTICS Strategy for obtaining comparability § Control group (Placebo) • Masking of treatment for patient / physician • Comparability of extraneous factors § Randomization • Random assignment of treatments • Comparability of prognostic factors § Blinding of the observer • Comparability of information on outcome 15/65

BIOSTATISTICS A. Controls - Comparability of extraneous factors - 16/65

BIOSTATISTICS Types of Controls § External • Historical • Concurrent, not randomized § Internal (concurrent, randomized) • • No treatment Placebo Dose-response Active control (positive control) § Multiple • Both an Active and Placebo • Multiple doses of test drug and of an active control 17/65

BIOSTATISTICS 1. Historical control § Treatment outcome을 previous series of comparable subjects와 비교 § Non-randomized, non-concurrent § Rapid, inexpensive, good for initial testing of new Tx § Two sources of historical control data • Literature (subject to publication bias) / database and/or registry § Problems • • Bias에 취약 New treatment의 효과를 과장하는 경향 Literature controls은 특히 poor 동일한 기관 내 동일한 과거 trial의 historical controls 역시 problematic 18/65

BIOSTATISTICS 2. Concurrent control § Not randomized § Patients compared, treated by different strategies, same period § Advantage • Eliminate time trend • Data of comparable quality § Disadvantage • Selection Bias • Treatment groups not comparable § Covariance analysis not adequate 19/65

BIOSTATISTICS 3. Placebo control § The “placebo effect” is well documented § Could be • Placebo alone (no other treatment) • Standard care + placebo § Matched placebos are of necessary • Pts & researchers should not decode Tx assignment § Randomized (concurrent) control • Considered as a Gold Standard 20/65

BIOSTATISTICS B. Randomization - Comparability of prognosis factors - 21/65
BIOSTATISTICS Comparability of prognosis § Randomization • Purpose − 동일한 특성을 지닌, 비교 가능한, 환자 군들을 생성 − Clinical evaluation에 관한 valid statistical tests를 보장 − Known and unknown risk factor들에 관한 비교성 확보 • Rationale − Treatment 처치 전에 prognostic factor들을 comparable하게 함 − 최상의 therapy를 찾을 수 있는 최선의 방법 − Current and future pts들이 harmful Tx를 받을 위험 최소화 • 집단들 간 비교가 연구 개시 시점부터 동일 선상에서 비 교될 수 있도록 보장하는 유일한 수단 – 따라서 selection bias 및 confounding bias 제거 • Allow RCT to play a key role in advancing medical science 22/65

BIOSTATISTICS § Randomization The success of randomization (무작위화) • Two inter-related processes 1. Random allocation (무작위 배정) − A random process로 allocation sequence 생성 − Allocation sequence의 unpredictability를 보장 2. Allocation concealment (무작위배정 은폐) − Trial에 involve된 사람들로 하여금 upcoming assignments를 파악하는 것을 방지 − 이에 대한 protection 없이는 investigator와 patient들은 누구 에게 다음 번 assignment가 이루어질 지 알게 될 것이고, 이 는 곧 group 간 동등한 comparison이 이루어질 수 없게 할 것 23/65

BIOSTATISTICS Randomization § 부적절한 무작위배정 은폐 예 • After creating an adequate allocation sequence using a random number table, affix the list to a bulletin board with no allocation concealment − Admitted patients could ascertain the upcoming treatment allocations − Route them with better prognoses to the intervention group and with poorer prognoses to the control group, or vice versa • Randomization list is posted on the web. • 해결 • Use an opaque sealed envelope • Use IVRS / IWRS randomization 24/65

BIOSTATISTICS Ethics of Randomization (1) § Biostatistician/clinical trialist must sell benefits of randomization § Physicians should do what he/she thinks is best for his patient • Two MD's might ethically treat same patient quite differently § Chalmers & Shaw (1970) • Annals New York Academy of Science 1. 2. 3. If MD "knows" best treatment, should not participate in trial If in doubt, randomization gives each patient equal chance to receive one of therapies (i. e. best) More ethical way of practicing medicine 25/65
BIOSTATISTICS Ethics of Randomization (2) § Byar et al. (1976) NEJM 1. RCT: honest admission that best is not known! 2. RCT is the best method to find out! 3. It reduces risk of being on inferior treatment 4. It reduces risk for future patients § Classic Example • Ref. Silverman (1977) Scientific Amer. 1. High dose oxygen to premature infants: common practice ↓ 2. Suspicion about frequency of blindness ↓ 3. RCT showed high dose cause of blindness 26/65
BIOSTATISTICS Types of randomization § Fixed allocation randomization • Simple randomization • Block randomization • Stratified randomization § Adaptive randomization • Minimization method • Covariate adaptive randomization – Baseline adaptive randomization – Response adaptive randomization 27/65
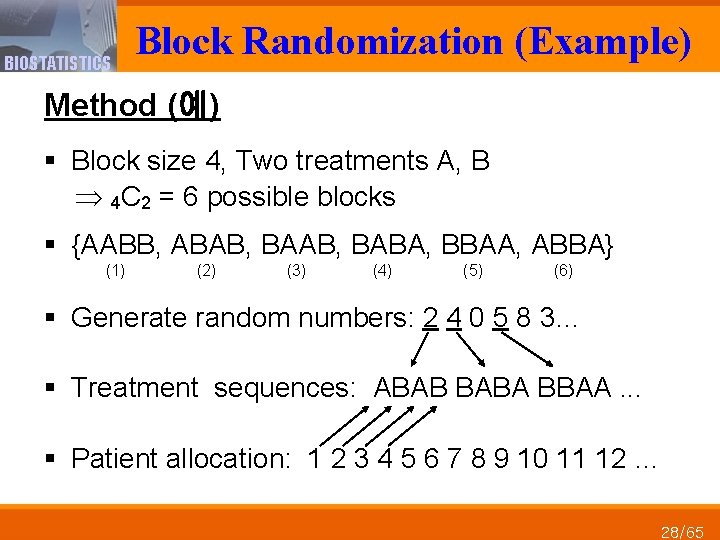
BIOSTATISTICS Block Randomization (Example) Method (예) § Block size 4, Two treatments A, B 4 C 2 = 6 possible blocks § {AABB, ABAB, BABA, BBAA, ABBA} (1) (2) (3) (4) (5) (6) § Generate random numbers: 2 4 0 5 8 3… § Treatment sequences: ABAB BABA BBAA. . . § Patient allocation: 1 2 3 4 5 6 7 8 9 10 11 12 … 28/65
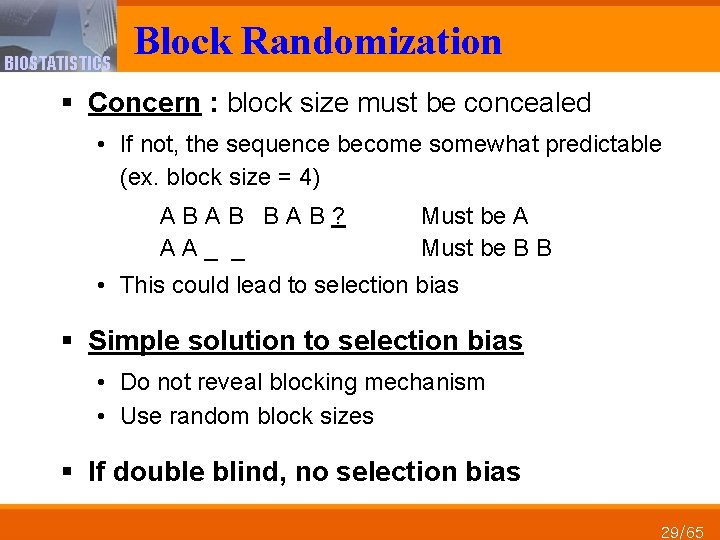
BIOSTATISTICS Block Randomization § Concern : block size must be concealed • If not, the sequence become somewhat predictable (ex. block size = 4) A B B A B ? A A _ _ Must be A Must be B B • This could lead to selection bias § Simple solution to selection bias • Do not reveal blocking mechanism • Use random block sizes § If double blind, no selection bias 29/65
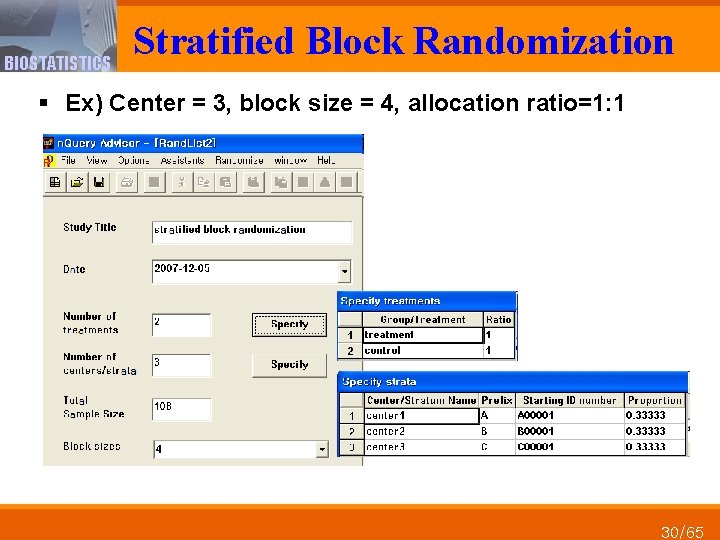
BIOSTATISTICS Stratified Block Randomization § Ex) Center = 3, block size = 4, allocation ratio=1: 1 30/65
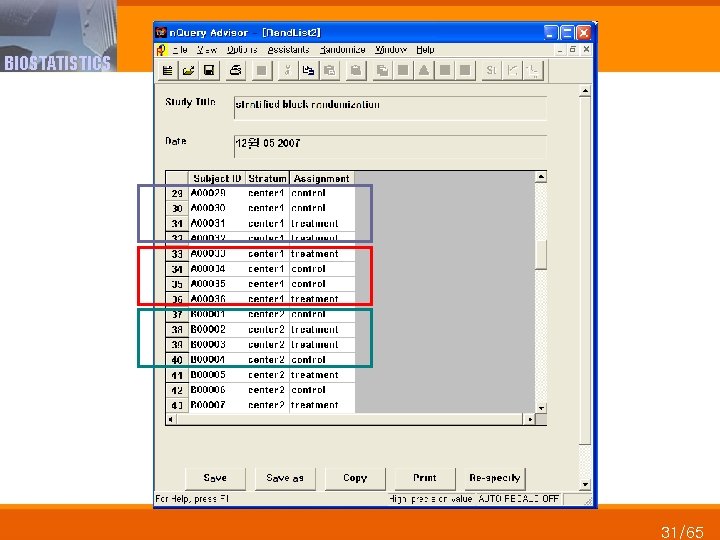
BIOSTATISTICS 31/65
Implementation - Timing BIOSTATISTICS § Actual randomization § Should be delayed until just prior to therapy initiation § Example • Alprenolol Trial, Ahlmark, et al. (1976) – – Nonblinded trial for AMI 393 patients randomized at the time of admission Alprenolol treatment was not initiated until 2 weeks later 231 excluded due to MI not documented, dead, contraindications to therapy, etc. – Only 162 patients treated, 69 alprenolol & 93 placebo – Imbalance raises concerns about comparability and/or possible bias for exclusion § Delaying randomization until initiation § Pt’s withdrawal로 인한 문제를 partly 해결해 줌 32/65

BIOSTATISTICS C. Blinding - Comparability of outcome information - 34/65
BIOSTATISTICS Comparability of information § 군 간 비교 시 risk of personal bias (information bias, detection bias)의 예방 필요 • Biased patient reporting • Biased ascertainment of information by physician • Biased assessment of information by physician, data manager, biostatistician § Solution: BLINDING (Masking) § Blinding의 목적: dual • To avoid bias during data collection and assessment − Comparability of information을 보장 • To implement placebo treatment − Comparability of extraneous effects를 보장 35/65

Types of blinding BIOSTATISTICS § Open label trials • Both patient and investigator know Tx assignment • 이를 해결하기 위한 design issue • PROBE design의 사용, OAC 운용 § Single blinding • When the investigator knows but the patient does not • Subjects masking § Double blinding • Neither patient nor investigator (HC provider) knows • Treatment team (include evaluator) masking § Triple blinding • Include project clinician, the biostatistician, the CRA, the programmer, and the data coordinator • Evaluation team masking 36/65

BIOSTATISTICS Types of Clinical Trials 37/65
BIOSTATISTICS Types of clinical trials § Prevention trial vs. Therapeutic trial • Preventing dz/recurrence vs. Treating dz • Differ in… complexity / recruitment strategies / compliance / length of follow-up / trial size, etc. § Confirmative trial vs. Pragmatic trial § Randomized trial vs. Non-randomized trial § Single center trial vs. Multi-center trial § Bioequivalence trial, Phase I, III trials § Superiority vs. Non-inferiority vs. Equivalence trial § Adaptive trials § Lots of other definitions and variants 38/65

BIOSTATISTICS Statistical Analysis 39/65
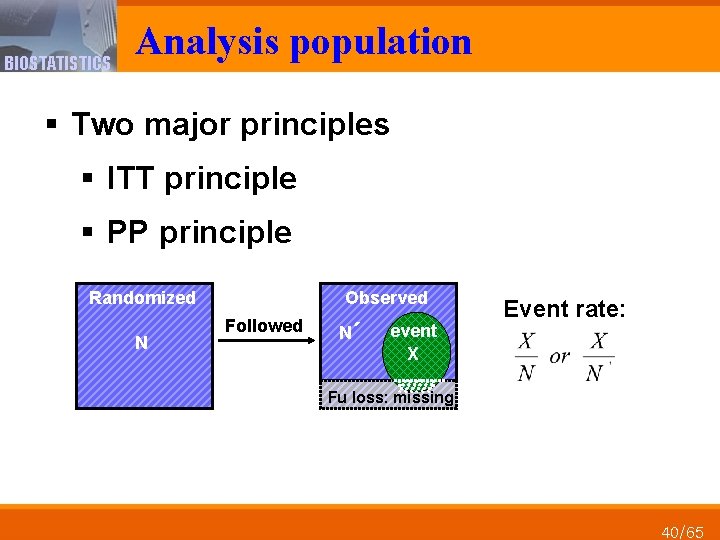
BIOSTATISTICS Analysis population § Two major principles § ITT principle § PP principle Randomized Observed Followed N N´ event X Event rate: Fu loss: missing N 40/65
BIOSTATISTICS 통계 분석 § Commonly used approaches • Intention-to-treat analysis (ITT analysis) − Full Analysis Set (FAS) − Modified ITT • As treated (On-treatment) analysis (AT/OT analysis) • Per-protocol analysis (PP analysis) § Missing data 처리 • LOCF approach (NOT GOOD) • Imputation (MI) • Use of a statistical method (ex. : “MMRM analysis”) 41/65

BIOSTATISTICS Dataset 42/65
Raw data (Source BIOSTATISTICS data) 43/65
BIOSTATISTICS Data Dictionary 44/65

BIOSTATISTICS Data Management 45/65
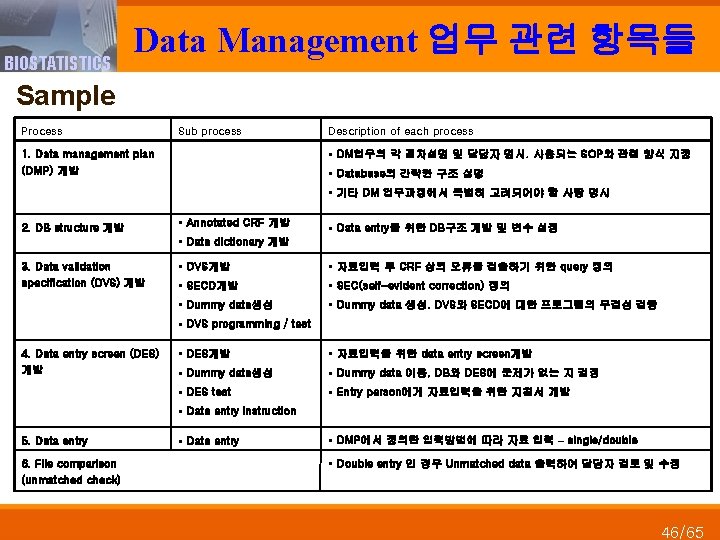
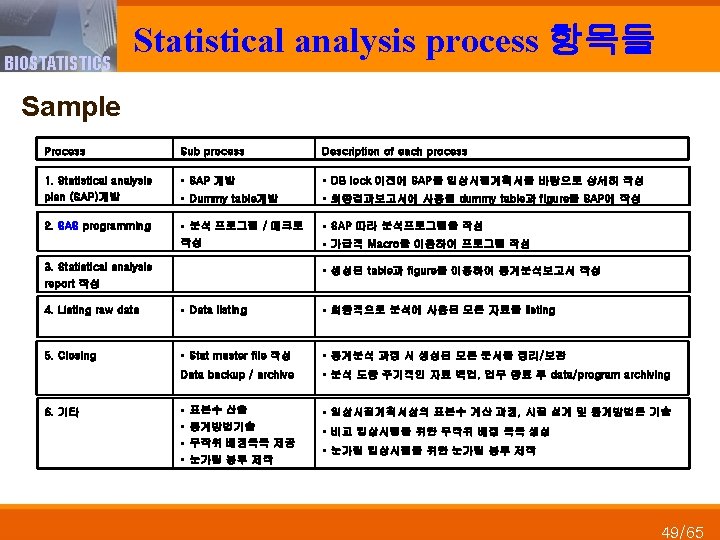
BIOSTATISTICS Data Management 업무 관련 항목들 Sample Process Sub process Description of each process § DM업무의 각 절차설명 및 담당자 명시. 사용되는 SOP와 관련 양식 지정 1. Data management plan (DMP) 개발 § Database의 간략한 구조 설명 § 기타 DM 업무과정에서 특별히 고려되어야 할 사항 명시 2. DB structure 개발 § Annotated CRF 개발 § Data entry를 위한 DB구조 개발 및 변수 설정 § Data dictionary 개발 3. Data validation specification (DVS) 개발 § DVS개발 § 자료입력 후 CRF 상의 오류를 검출하기 위한 query 정의 § SECD개발 § SEC(self-evident correction) 정의 § Dummy data생성 § Dummy data 생성. DVS와 SECD에 대한 프로그램의 무결성 검증 § DVS programming / test 4. Data entry screen (DES) 개발 § DES개발 § 자료입력을 위한 data entry screen개발 § Dummy data생성 § Dummy data 이용, DB와 DES에 문제가 없는 지 검정 § DES test § Entry person에게 자료입력을 위한 지침서 개발 § Data entry instruction 5. Data entry 6. File comparison (unmatched check) § Data entry § DMP에서 정의한 입력방법에 따라 자료 입력 – single/double § Double entry 인 경우 Unmatched data 출력하여 담당자 검토 및 수정 46/65
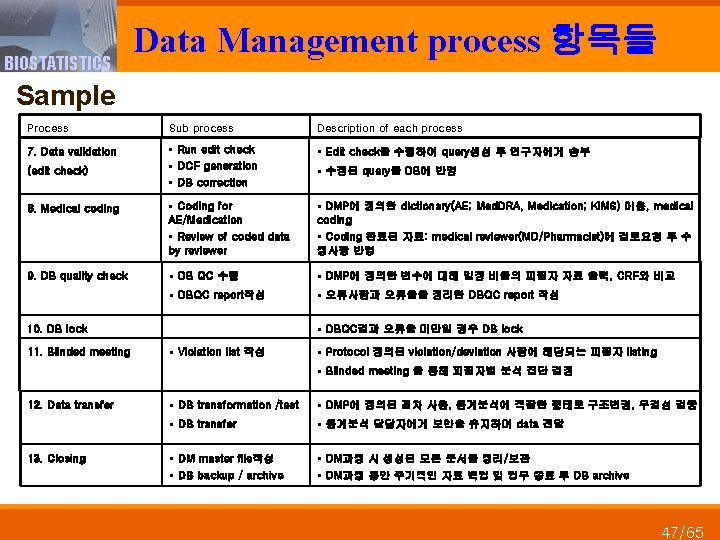
BIOSTATISTICS Data Management process 항목들 Sample Process Sub process Description of each process 7. Data validation § Run edit check § DCF generation § DB correction § Edit check을 수행하여 query생성 후 연구자에게 송부 8. Medical coding § Coding for AE/Medication § Review of coded data by reviewer § DMP에 정의한 dictionary(AE; Med. DRA, Medication; KIMS) 이용, medical coding § Coding 완료된 자료: medical reviewer(MD/Pharmacist)에 검토요청 후 수 정사항 반영 9. DB quality check § DB QC 수행 § DMP에 정의한 변수에 대해 일정 비율의 피험자 자료 출력, CRF와 비교 § DBQC report작성 § 오류사항과 오류율을 정리한 DBQC report 작성 (edit check) § DBQC결과 오류율 미만일 경우 DB lock 10. DB lock 11. Blinded meeting § 수정된 query를 DB에 반영 § Violation list 작성 § Protocol 정의된 violation/deviation 사항에 해당되는 피험자 listing § Blinded meeting 을 통해 피험자별 분석 집단 결정 12. Data transfer 13. Closing § DB transformation /test § DMP에 정의된 절차 사용, 통계분석에 적합한 형태로 구조변경, 무결성 검증 § DB transfer § 통계분석 담당자에게 보안을 유지하며 data 전달 § DM master file작성 § DB backup / archive § DM과정 시 생성된 모든 문서를 정리/보관 § DM과정 동안 주기적인 자료 백업 및 업무 종료 후 DB archive 47/65

BIOSTATISTICS Data Management Plan (DMP) 48/65

BIOSTATISTICS Statistical Analysis Plan (SAP) 50/65

BIOSTATISTICS Sample Size 51/65

BIOSTATISTICS u v 임상시험 시작 시 적정 피험자 수 산출 적절하게 계획되고 수행된 clinical trial • v Why should we calculate the sample size? Intervention의 effectiveness를 평가할 수 있는 powerful experimental technique 임상시험은 군 간 차이를 detect할 수 있는, 충분한 크기의 statistical “power”가 확보되어야 • Under-powered trial은 이후 다시 시도되지 않을 것 • Sample size 계산은 planning의 essential part v Sample size 계산을 위해서는 해당 study의 shape이 결정되어야 v Sometimes, sample size calculation is all we have to do to plan the study 52/65

BIOSTATISTICS Sample ☻ 53/65

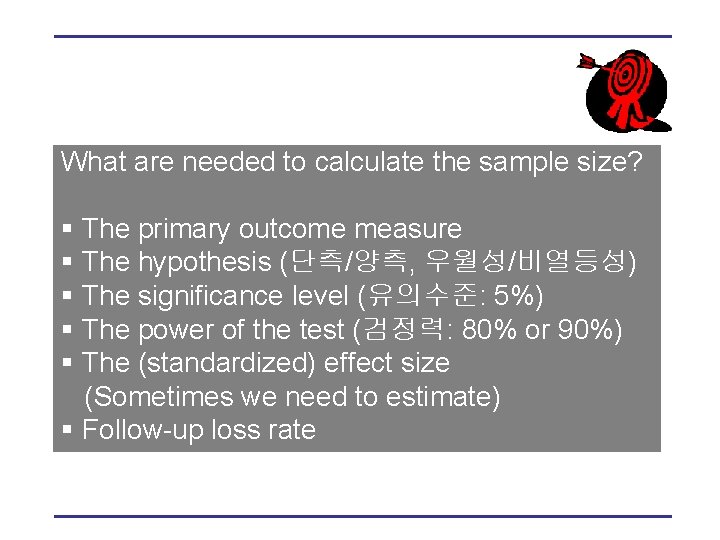
What are needed to calculate the sample size? § The primary outcome measure § The hypothesis (단측/양측, 우월성/비열등성) § The significance level (유의수준: 5%) § The power of the test (검정력: 80% or 90%) § The (standardized) effect size (Sometimes we need to estimate) § Follow-up loss rate
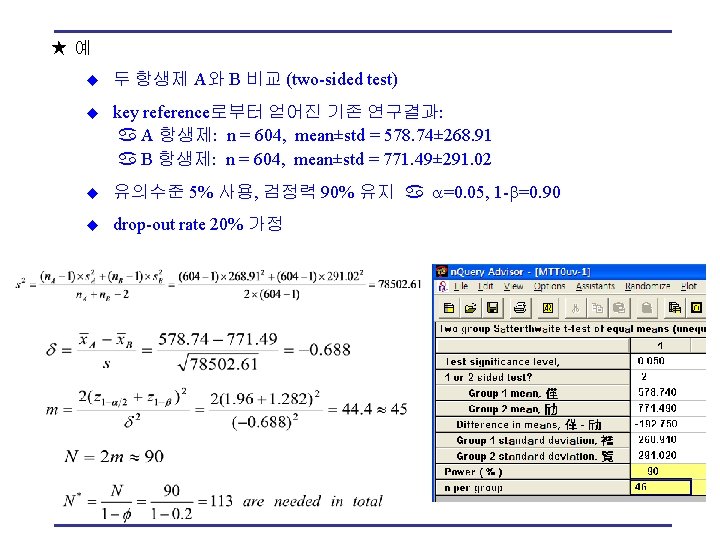
★예 u 두 항생제 A와 B 비교 (two-sided test) u key reference로부터 얻어진 기존 연구결과: A 항생제: n = 604, mean±std = 578. 74± 268. 91 B 항생제: n = 604, mean±std = 771. 49± 291. 02 u 유의수준 5% 사용, 검정력 90% 유지 =0. 05, 1 - =0. 90 u drop-out rate 20% 가정
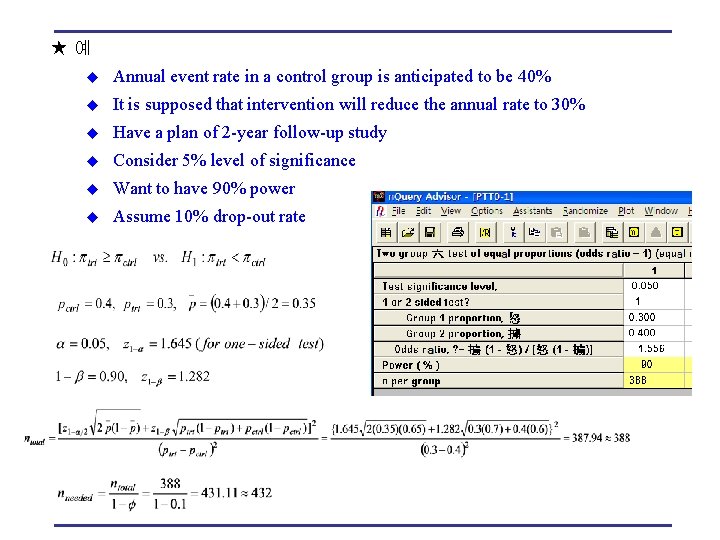
★예 u Annual event rate in a control group is anticipated to be 40% u It is supposed that intervention will reduce the annual rate to 30% u Have a plan of 2 -year follow-up study u Consider 5% level of significance u Want to have 90% power u Assume 10% drop-out rate
BIOSTATISTICS Role of Biostatistics 58/65
BIOSTATISTICS § § Role of Biostatistics In the design of the study In conduct and monitoring of the study In the analysis In interpretation of results 59/65
BIOSTATISTICS Biostatistics in Design § Good statistical planning은 absolutely essential § Study type에 대한 계획: 하나 이상의 design 가능 § 무엇을 볼 것인가? Superiority, NI, equivalence § Primary endpoint(s)는? § 가설(귀무가설, 대립가설)을 endpoints의 형태로 정의 § Bias, precision의 고려 § Control, design, blinding, randomization type들 고려 § 모집단과 관련된 issues들 (gender, age, sex 등) § Power and sample size determination 60/65

BIOSTATISTICS Biostatistics in Analysis § Trial conduct에 관한 plan • Non-compliance, missing data • Interim analysis를 고려하는 경우, stopping rules § Statistical analysis에 관한 고려 • Data transfer • Statistical Analysis Plan (SAP) 준비 • Multiplicities (endpoints, treatments, analyses)에 관한 고려 • Outliers, violation of assumptions, missing data handling • Multicenter analysis • Subgroup analysis 61/65
BIOSTATISTICS Biostatistics in Monitoring § 모든 participants들을 aggressive하게 follow-up • To minimize the amount of missing data § Pre-planned interim analysis • To monitor a study • Perhaps stop early § Re-sizing the trial • Planned in advance 62/65
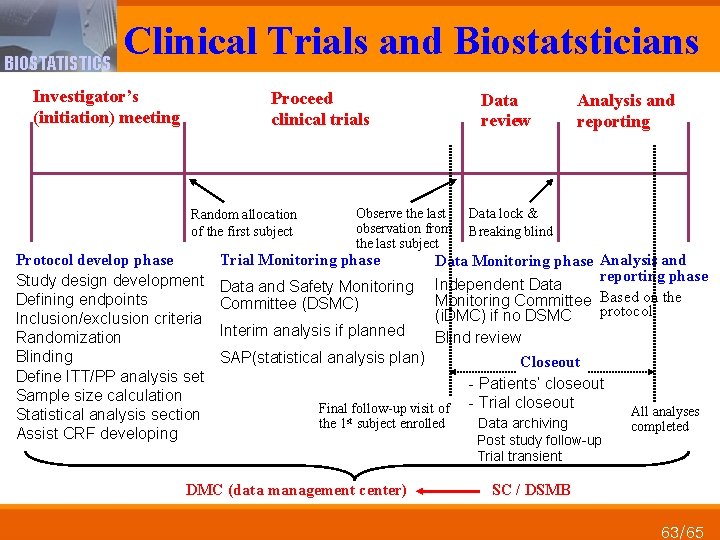
Clinical Trials and Biostatsticians BIOSTATISTICS Investigator’s (initiation) meeting Proceed clinical trials Random allocation of the first subject Protocol develop phase Study design development Defining endpoints Inclusion/exclusion criteria Randomization Blinding Define ITT/PP analysis set Sample size calculation Statistical analysis section Assist CRF developing Data review Observe the last observation from the last subject Trial Monitoring phase Data and Safety Monitoring Committee (DSMC) Interim analysis if planned Data lock & Breaking blind Data Monitoring phase Analysis and reporting phase Independent Data Monitoring Committee Based on the protocol (i. DMC) if no DSMC Blind review SAP(statistical analysis plan) Final follow-up visit of the 1 st subject enrolled DMC (data management center) Analysis and reporting Closeout - Patients’ closeout - Trial closeout Data archiving Post study follow-up Trial transient All analyses completed SC / DSMB 63/65

BIOSTATISTICS Conclusion 64/65
BIOSTATISTICS Conclusion u Clinical trial should have sufficient statistical power to detect differences between groups. A calculation of sample size is an essential part of planning. u Relevant baseline data should be measured before the start of intervention. u Once a participant is enrolled, taking measures to enhance and monitor subjects’ compliance is essential u During all phases of a study, sufficient effort should be spent to ensure data high quality. u Appropriate data monitoring and proper statistical analysis should not be underestimated to ensure the quality of clinical trial. 65/65
- Slides: 65