Biosimilars Marketed Enbrel Biosimilars Shangai CP Guojian Pharmaceutical
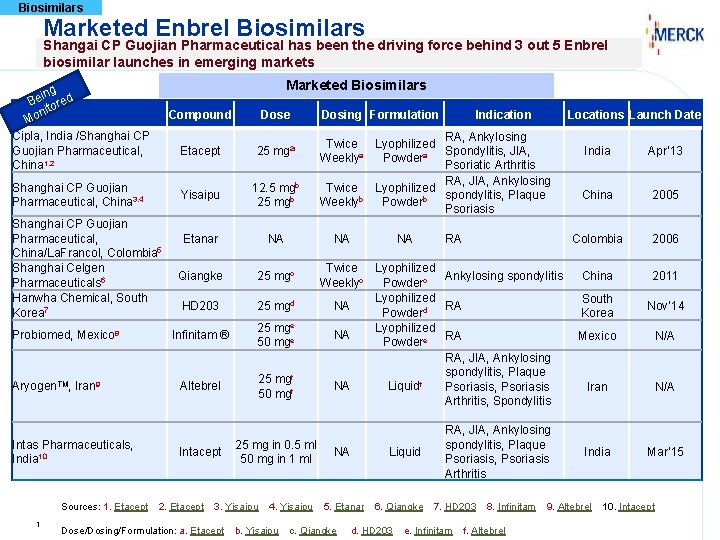
Biosimilars Marketed Enbrel Biosimilars Shangai CP Guojian Pharmaceutical has been the driving force behind 3 out 5 Enbrel biosimilar launches in emerging markets Marketed Biosimilars ing Be ored nit Mo Cipla, India /Shanghai CP Guojian Pharmaceutical, China 1, 2 Shanghai CP Guojian Pharmaceutical, China 3, 4 Shanghai CP Guojian Pharmaceutical, China/La. Francol, Colombia 5 Shanghai Celgen Pharmaceuticals 6 Hanwha Chemical, South Korea 7 Probiomed, Mexico 8 Aryogen. TM, Iran 9 Intas Pharmaceuticals, India 10 Sources: 1. Etacept 1 Compound Dose Etacept 25 mga Yisaipu 12. 5 mgb 25 mgb Etanar NA Qiangke 25 mgc HD 203 25 mgd Infinitam ® 25 mge 50 mge Altebrel 25 mgf 50 mgf Intacept 2. Etacept Dose/Dosing/Formulation: a. Etacept 4. Yisaipu b. Yisaipu Indication Locations Launch Date RA, Ankylosing Twice Lyophilized Spondylitis, JIA, Weeklya Powdera Psoriatic Arthritis RA, JIA, Ankylosing Twice Lyophilized spondylitis, Plaque Weeklyb Powderb Psoriasis NA 25 mg in 0. 5 ml 50 mg in 1 ml 3. Yisaipu Dosing Formulation NA RA Twice Lyophilized Ankylosing spondylitis Weeklyc Powderc Lyophilized NA RA Powderd Lyophilized NA RA Powdere RA, JIA, Ankylosing spondylitis, Plaque NA Liquidf Psoriasis, Psoriasis Arthritis, Spondylitis NA Liquid 5. Etanar c. Qiangke 6. Qiangke d. HD 203 RA, JIA, Ankylosing spondylitis, Plaque Psoriasis, Psoriasis Arthritis 7. HD 203 e. Infinitam 8. Infinitam f. Altebrel India Apr’ 13 China 2005 Colombia 2006 China 2011 South Korea Nov’ 14 Mexico N/A Iran N/A India Mar’ 15 9. Altebrel 10. Intacept
- Slides: 1