Bioprocess Engineering Kinetics Biosystems Sustainability and Reactor Design
Bioprocess Engineering Kinetics, Biosystems, Sustainability and Reactor Design Shijie Liu Chapter 1 Introduction

Renewable: Carbon recycled CO 2 H 2 O Energy (CH 2 O)n O 2 Introduction 2
Course General Info • Instructor: Prof. Shijie Liu 302 Walters Hall SUNY ESF sliu@esf. edu TA: Yang Wang 410 Walters Hall ywang 52@syr. edu • Course Materials: Class notes. • Textbook: Bioprocess Engineering – Kinetics, Biosystems, Sustainability and Reactor Design Introduction 3
General Course Info • General Description – Overview of Microbiology, product potential – Kinetics: biotransformation and chemical transformation – Ideal batch, continuous and fed-batch reactors – Bioreactor performance evaluation / design – Immobilized cell systems, mass transfer effects – Stability and Scale up – Genetically engineered organisms • Lectures, in-class exercises / discussions, assignments / homework, term paper/presentation (BPE 621), exams Introduction 4
Introduction • • Biological Processes What is Bioprocess Engineering Why Bioreaction Engineering What sets Bioprocess Engineers apart from other engineers and chemists and/or biologists – Chemical transformation and biotransformation – Processes for converting renewable raw materials – Biologics – Engineering biosystems in industrial applications Introduction 5

Biological Processes Introduction 6

Green Chemistry • 1). Developing products and processes based on renewable resources, such as plant biomass; • 2). Design processes that bypass dangerous / toxic chemical intermediates; • 3). Design processes that avoid dangerous / toxic solvent use; • 4). Reducing chemical processing steps, but demands high efficiency; • 5). Promoting biochemical processes to reduce chemical processing steps or toxic chemical utilization; • 6). Design milder (closer to atmosphere temperature and pressure) processes and multiple product recovery routes; • 7). Bypassing chemical equilibrium with innovative reactor design, and biocatalysis, rather than adding intermediate steps. • 8). Avoid production of unwanted products other than H 2 O and CO 2. Introduction 7
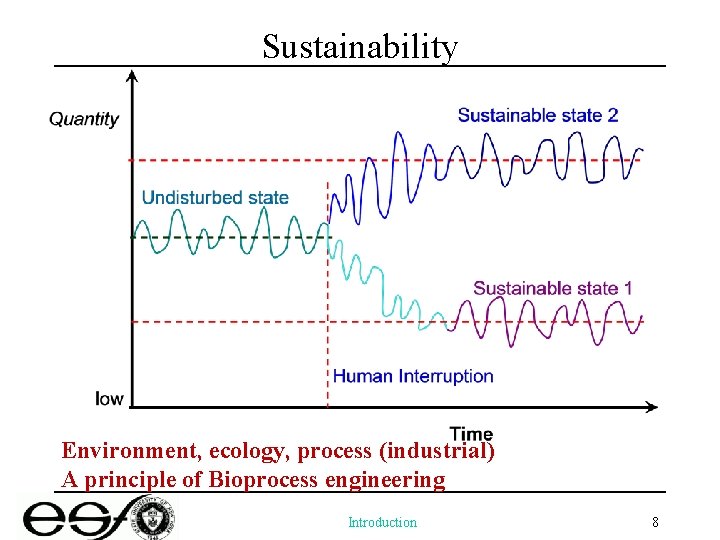
Sustainability Environment, ecology, process (industrial) A principle of Bioprocess engineering Introduction 8
Biorefinery Biological Basics 9
Bioprocess Engineering • Profession – Oversee process operations (process engineer) – Process (especially bioprocess) performance evaluation – Bioprocess operation optimization – Process Control – Bioprocess Design • Knowledge Base – Transport Phenomena / Bioseparations – (Bio)Reaction Engineering – Process Characterization (control) Biological Basics 10

Bio. Process Engineering Profession Bio. Process Engineering Transport Phenomena Mass Transfer Physics Mathematics Chemistry Specialization Momentum Transfer Heat Transfer Physics Mathematics Thermodynamics Physics Mathematics Pulp and Paper Oil Refining Mining, metal refining Cement Food Processing Biologics … Chemistry Physics or Biology Introduction Chemical/Bio Reaction Chemical Reaction Thermodynamics Physical Chemistry Mathematics Bio Reaction Biology Chemistry Mathematics 11
Regulatory Issues Biologics such as vaccines and recombinant protein drugs are to be approved by FDA. There are five phases of development after discovery: 0). Human microdosing studies (1 -3 years). Exploratory 1). First stage testing in human subjects (~ 1 year). 2080 healthy volunteers 2). Clinical trials (~ 2 years). 100 -300 patients and volunteers 3). Large clinical trials (~ 3 years). 300 – 3000 + patients 4). Post marketing surveillance trials Introduction 12
Learning • Life-time commitment • Self-motivation • Use of internet / Library – Source of knowledge Introduction 13
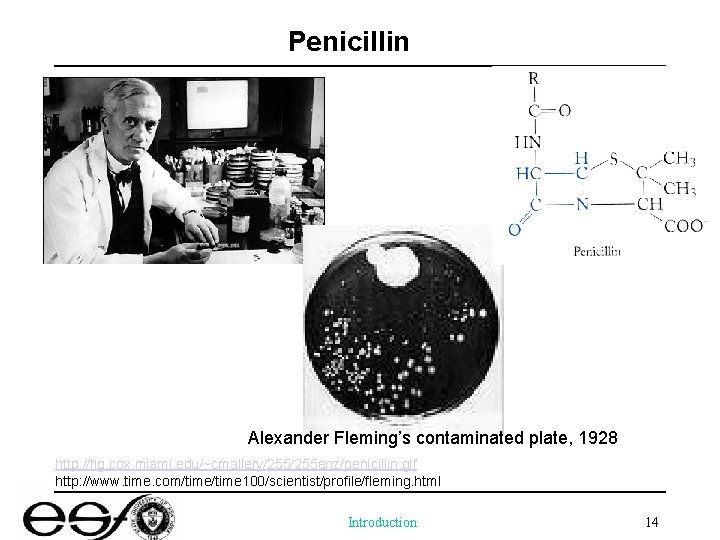
Penicillin Alexander Fleming’s contaminated plate, 1928 http: //fig. cox. miami. edu/~cmallery/255 enz/penicillin. gif http: //www. time. com/time 100/scientist/profile/fleming. html Introduction 14

A milestone in the history of human medicine by Alexander Fleming in 1928 • An accidental discovery of an antibiotic produced by a common mold of Penicillium notatum • High demand of antibiotics during World War II • Norman Heatley, a biochemist, optimized the culture condition and recovery of the drug for animal and human tests • Promising results obtained, but the material was exhausted resulting the death of a patient Introduction 15

How could penicillin be produced in large scale? ? • US became the major player due to the absence of a war • Merck, Pfizer, Squibb, and USDA Northern Regional Research Lab (USDA-NRRL) • First idea (1940 s)-chemical synthesis, turned out to be extremely difficult Introduction 16

Produce penicillin by fermentation Major problem: low concentration of the product complicates the purification process (0. 001 g/L in 1939) Solutions: • A new corn steep liquor-lactose based medium developed by USDA-NRRL (10 x increase in yield) • A better strain for producing penicillin: Penicillium chrysogenum • Fermentation process: change from surface method (“bottle plant”) to submerged process – challenges in knowledge of mold physiology and tank design/control (sterilization, oxygen supply, heat removal) • Purification: Penicillin, as most of the biological products, is fragile (p. H shifts and liquid-liquid extraction) Introduction 17
How was this archived • Merck’s model: partnership of biologists and chemical engineers an interdisciplinary approach • 0. 001 g/L (1939) ----- 50 g/L (1990) • The birth of the concept of bioprocess engineering Introduction 18
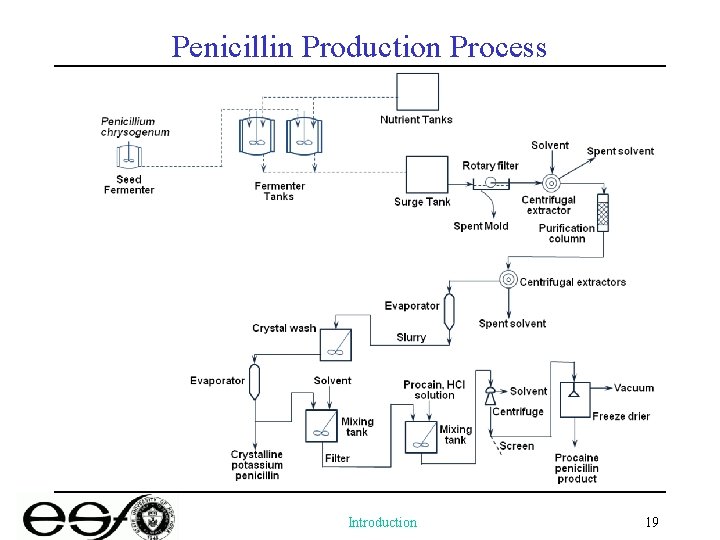
Penicillin Production Process Introduction 19
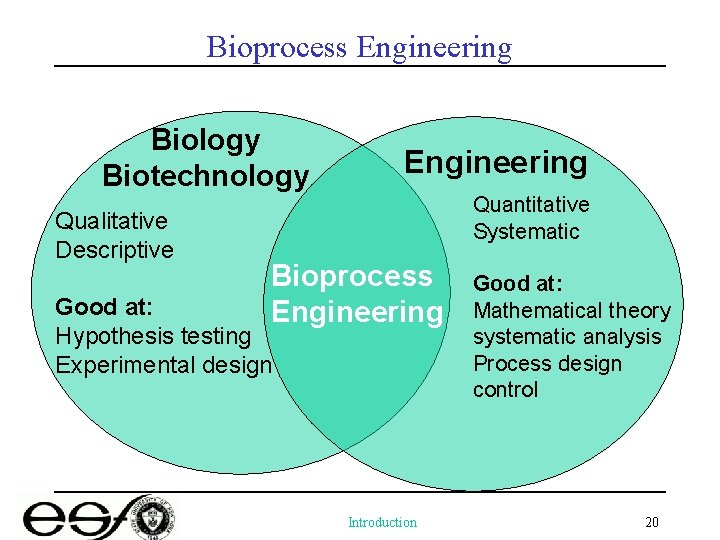
Bioprocess Engineering Biology Biotechnology Qualitative Descriptive Engineering Quantitative Systematic Bioprocess Engineering Good at: Hypothesis testing Experimental design Introduction Good at: Mathematical theory systematic analysis Process design control 20
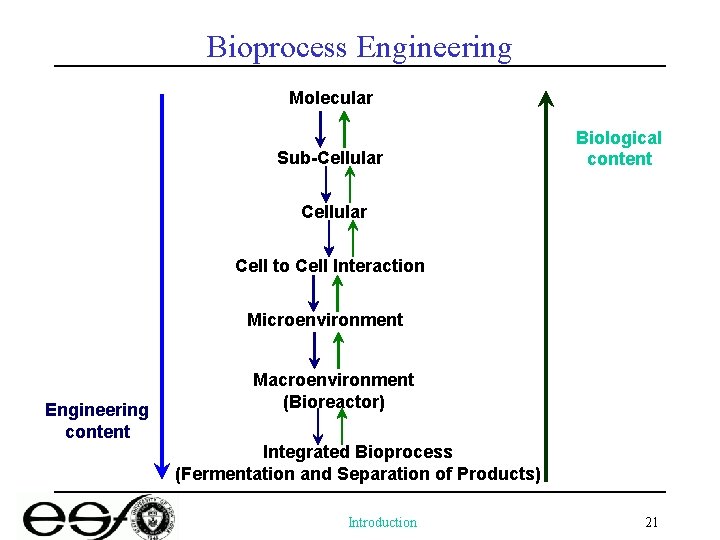
Bioprocess Engineering Molecular Sub-Cellular Biological content Cellular Cell to Cell Interaction Microenvironment Engineering content Macroenvironment (Bioreactor) Integrated Bioprocess (Fermentation and Separation of Products) Introduction 21
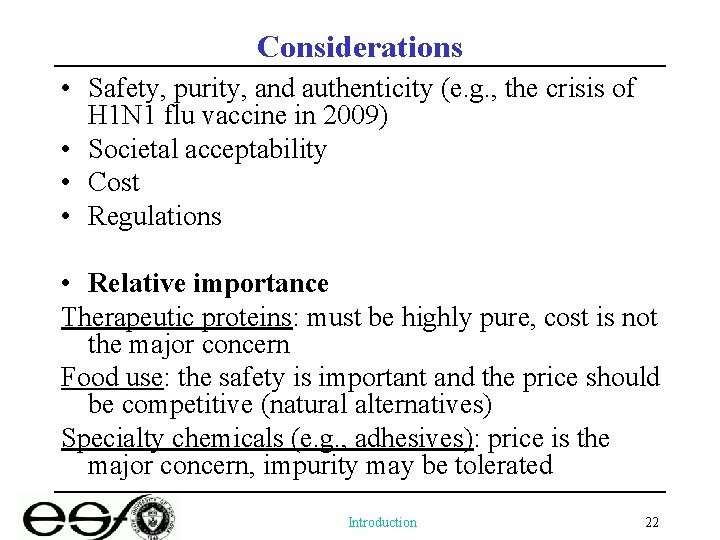
Considerations • Safety, purity, and authenticity (e. g. , the crisis of H 1 N 1 flu vaccine in 2009) • Societal acceptability • Cost • Regulations • Relative importance Therapeutic proteins: must be highly pure, cost is not the major concern Food use: the safety is important and the price should be competitive (natural alternatives) Specialty chemicals (e. g. , adhesives): price is the major concern, impurity may be tolerated Introduction 22
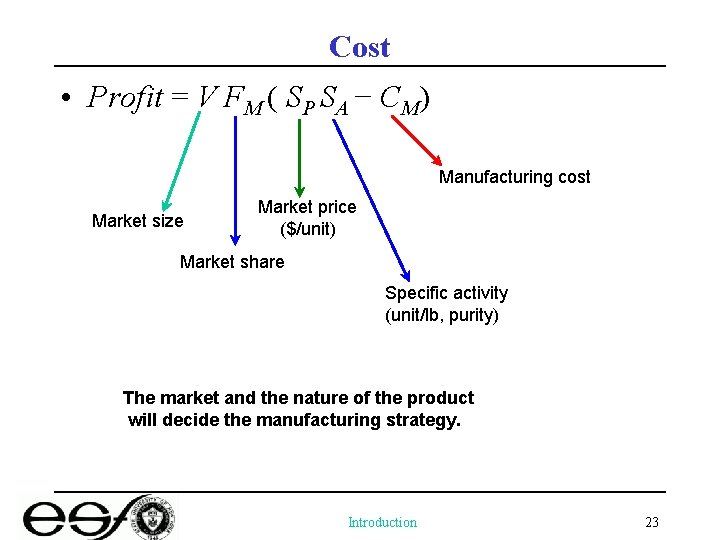
Cost • Profit = V FM ( SP SA − CM) Manufacturing cost Market size Market price ($/unit) Market share Specific activity (unit/lb, purity) The market and the nature of the product will decide the manufacturing strategy. Introduction 23
How many decisions need to be made? • Expression system (host, vector) FDA • Type and the size of the reactors • Culturing conditions (media, p. H, temperature, mixing. . ) EPA • Mode of operation • Downstream purification OSHA • Quality control • Regulation USDA Introduction Competitors 24

Quality Control • • GMP: Good manufacturing practice GLP: Good laboratory practices SOP: standard operating procedure Validation of computer software Introduction 25

Summary Sustainability Environmental, Ecological Biorefinery Sustainable Green chemistry Environmental, sustainable Bioprocess Engineering Safety, Quality, Optimum Regulatory Social acceptability, accountability Environmental Introduction 26
- Slides: 26