Biopharmacy drug absorption mechanisms of absorption physicochemical factors
Biopharmacy drug absorption; mechanisms of absorption; physicochemical factors related
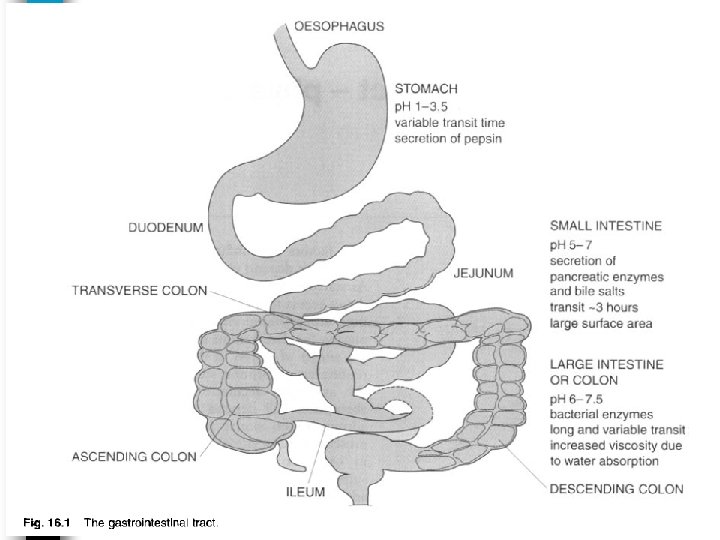

The systemic drug absorption from GIT or from any other extravascular site is dependent on : 1 - The physicochemical properties of the drug. 2 - Pharmaceutical and formulatory factors. 3 - Anatomy and physiology of absorption site (GIT).

Pharmaceutical and formulatory factors

• The type of dosage form can influence the bioavailability of the drug. • Thus whether a drug is incorporated in the form of solution, suspension, or solid dosage form (tablet or capsule) can influences its rate and/or extent of absorption from GIT.

In general, drug to be absorbed from GIT they must be in solution form. Thus the greater the number of intervening steps to be in solution (disintegration then dissolution) the greater will be the number of potential obstacles to absorption. Hence; The bioavailability of a given drug tends to decrease in the following order of the types of dosage form for same drug

Aqueous solution > Aqueous suspension > Solid dosage form
Influence of the Type of Dosage Form Aqueous Solution : Factors associated with formulation of aqueous solution that can influence drug bioavailability include: • The chemical stability of the drug in aqueous solution and gastric fluid, • complexation of drug with the excepients included in the solution, • incorporation of surface active agents to increase water solubility of the drug and • incorporation of viscosity enhancer.

Aqueous Suspension : It is a useful dosage form for administrating an insoluble or poorly water soluble drug. Usually the absorption of the drug from this type of dosage form is dissolution rate limited. The oral administration of an aqueous suspension results in a large total surface area of the dispersed drug being immediately presented to the GI fluids comparing with solid oral dosage forms. This facilitates dissolution and hence absorption of the drug.

Factors associated with formulation of aq. suspension dosage forms that can influence the F of a drug from GIT include: • The particle size and • effective surface area of the dispersed drug, • crystal form of the drug, • formation of a non-absorbable complex, • • the inclusion of surfactants as wetting, • flocculating or deflocculating agent, and • the viscosity of the suspension

Capsule : Generally the bioavailability of a drug from a well formulated powder filled hard gelatin capsule dosage form will be better than or at least equal to that for the same drug in a compressed tablet.
The inclusion of hydrophilic or hydrophobic diluents will effect the dissolution rate of drugs encapsulated. hydrophilic diluents (e. g sorbitol, lactose) often serve to increase the rate of penetration of the aqueous GI fluids into contents of the capsule.

An increasing in packing density (e. g decrease in porosity) of the encapsulated mass will probably result in a decrease in liquid permeability and dissolution rate, particularly if : Drug is hydrophobic or if hydrophilic drug is mixed with hydrophobic lubricant such as magnesium stearate, inclusion of surfactant will be valuable in this case to increase liquid penetration

Formulation factors that can influence drug bioavailability from hard gelatin capsules include: • • particle size and surface area, the use of salt form crystal form of the drug, chemical stability, the nature and the quantity of diluents especially lubricant and surfactants, and • type and condition of filling process

Tablet : most widely used dosage form n there is an enormous reduction in the effective surface area. n a poorly soluble drug VS. tablets containing such drugs should disintegrate rapidly and completely if rapid release, dissolution and absorption are required. n
Formulation factors that can influence drug bioavailability from uncoated tablet wettability, effective surface area, crystal form, chemical stability, n Nature and quantity of the diluent, binder, disintegrants, lubricant and wetting agents, n Compaction pressure and speed of compaction used in tabletting n conditions of storage and age of the tab. n

For coated tablets, the presence of coating presents a physical barrier between tablet core and GI fluids: problems associated with coated tablets & are subject to the additional potential problem of being surrounded by physical barrier n The physicochemical and thickness of the coating can thus influence how quickly a drug is released from tablet n

• Hydroxypropylmethyl cellulose (HPMC) a water soluble polymer (has no effect), • Ethyl cellulose a water insoluble polymer, delay and/or reduce the rate of drug release • Coating particularly Shellac, will crosslink upon aging and decrease the dissolution rate

• For enteric coated tablets • defect in the formulation will affect the extent of intact drug absorbed.

Influence of Excepients for Conventional Dosage Forms n Excepients include disintegrants, diluents, lubricants, suspending agents, emulsifying agents, flavoring agents, coloring agents …. etc, n must be inert in that they should exert no therapeutic or biological action, or modify the biological action of the drugs present in the dosage forms.

Diluents : Some diluents had been reported to be responsible for decreasing the gastrointestinal absorption of drug because part of the administered dose of the drug formed a poorly absorbable complex with the diluents and hence decrease bioavailability

Disintegrants : n Disintegrants are required to break up capsules, tablets and granules into primary powder particles in order to increase the surface area of the drug exposed to GI fluids. n n A tablet that fail to disintegrate or disintegrate slowly may result in incomplete absorption or a delay in the onset of action of the drug

Lubricants : Both tablets and capsules require lubricants in their formulation to reduce friction between the powder and metal surfaces during their manufacturing. Lubricants are often hydrophobic in nature (e. g magnesium stearate) and retards liquids penetration into capsules or tablets ingredients, this problem can usually overcome by simultaneous addition of wetting agent ( e. g water soluble surfactants) and the use of hydrophilic diluents.



Thank you
- Slides: 26