BiomoleculesMacromolecules Nucleic Acids Carbohydrates Inorganic Compounds that do
Biomolecules/Macromolecules Nucleic Acids Carbohydrates

Inorganic – Compounds that do not have chains of carbon and are not created by life/living things. Ammonia Salt Water
Organic – Compounds that are created by life and contain chains of carbon atoms. Macromolecules are large organic molecules
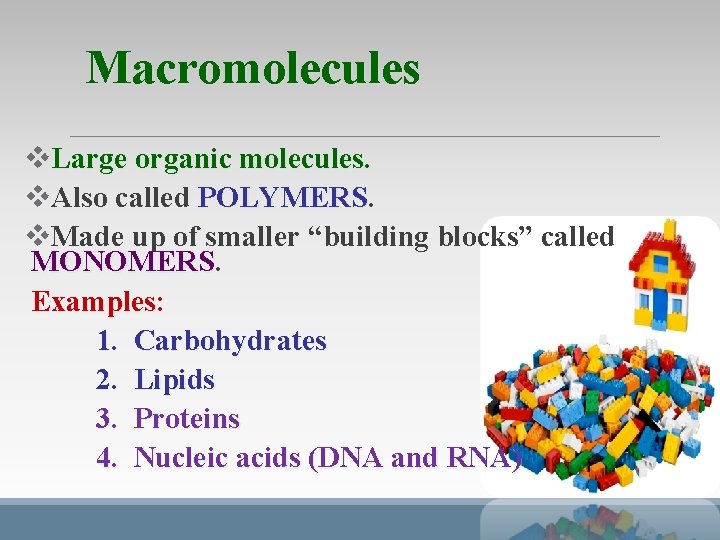
Macromolecules v. Large organic molecules. v. Also called POLYMERS v. Made up of smaller “building blocks” called MONOMERS Examples: 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic acids (DNA and RNA)
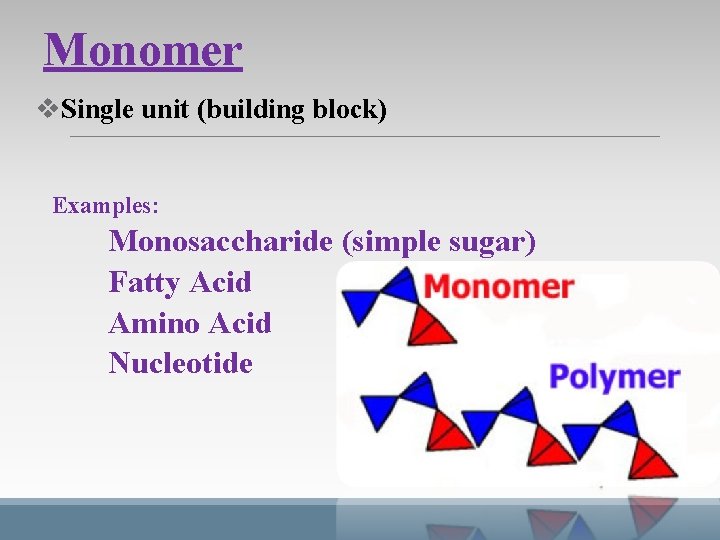
Monomer v. Single unit (building block) Examples: Monosaccharide (simple sugar) Fatty Acid Amino Acid Nucleotide
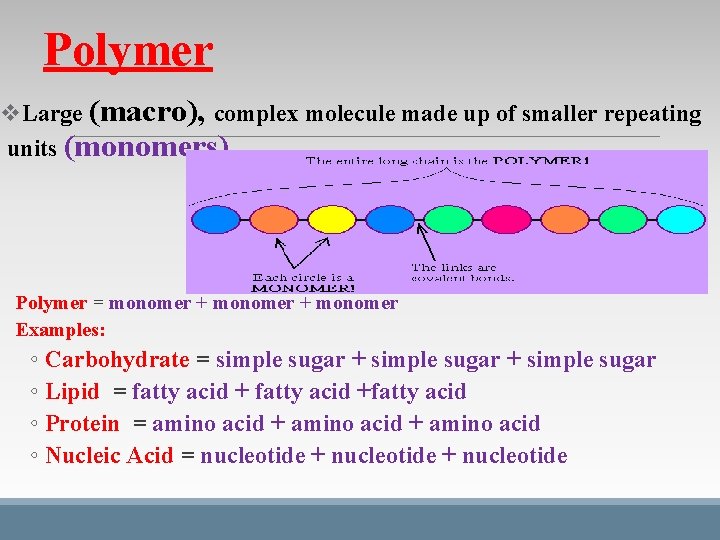
Polymer v. Large (macro), complex molecule made up of smaller repeating units (monomers) Polymer = monomer + monomer Examples: ◦ Carbohydrate = simple sugar + simple sugar ◦ Lipid = fatty acid +fatty acid ◦ Protein = amino acid + amino acid ◦ Nucleic Acid = nucleotide + nucleotide

Question: How Are Macromolecules Formed?
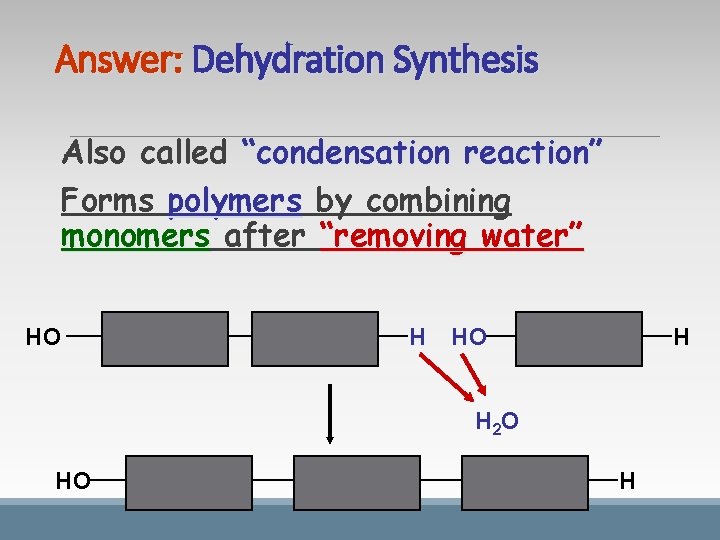
Answer: Dehydration Synthesis Also called “condensation reaction” Forms polymers by combining monomers after “removing water” HO H H 2 O HO H

Question: How are Macromolecules separated or digested?
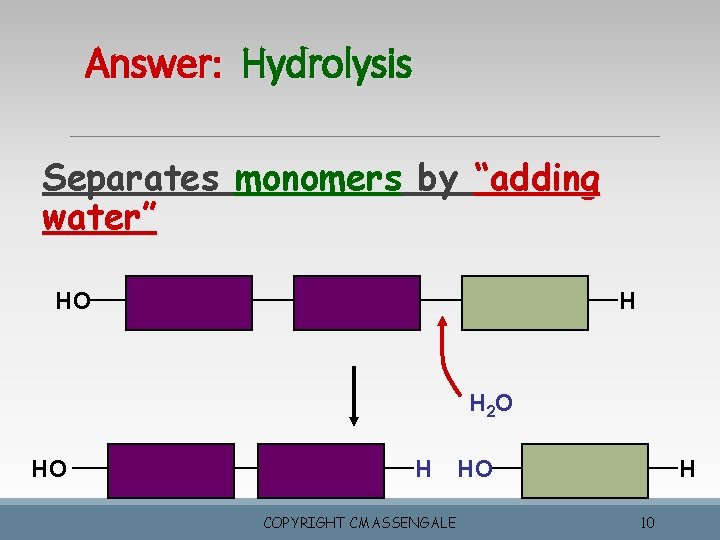
Answer: Hydrolysis Separates monomers by “adding water” HO H H 2 O HO H COPYRIGHT CMASSENGALE HO H 10
4 Main Classes of Organic Compounds 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Also called: Biomolecules “life” molecules Macromolecules “large” molecules
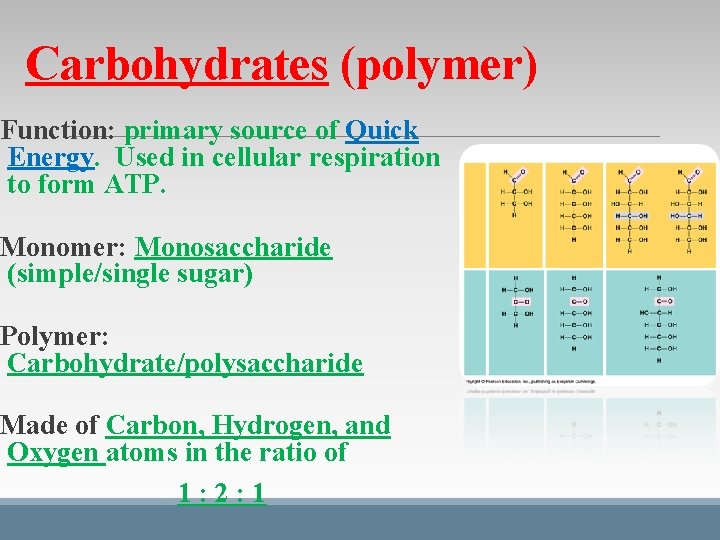
Carbohydrates (polymer) Function: primary source of Quick Energy. Used in cellular respiration to form ATP. Monomer: Monosaccharide (simple/single sugar) Polymer: Carbohydrate/polysaccharide Made of Carbon, Hydrogen, and Oxygen atoms in the ratio of 1: 2: 1

Fruits and Vegetables

TREES, PLANTS, &
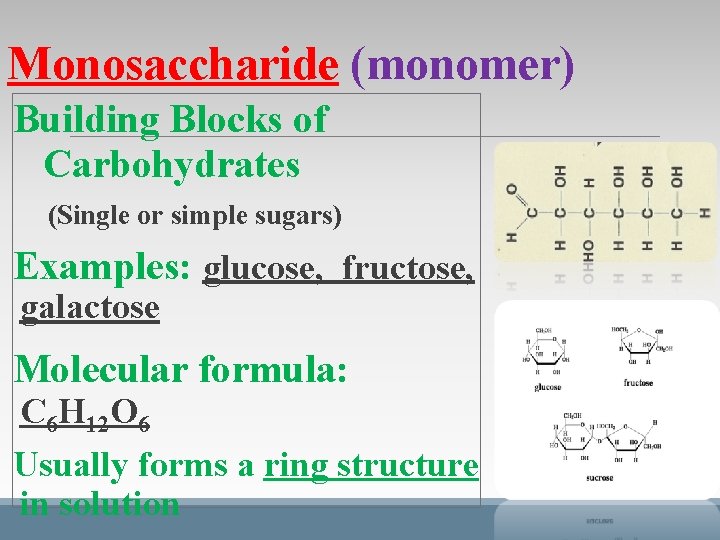
Monosaccharide (monomer) Building Blocks of Carbohydrates (Single or simple sugars) Examples: glucose, fructose, galactose Molecular formula: C 6 H 12 O 6 Usually forms a ring structure in solution
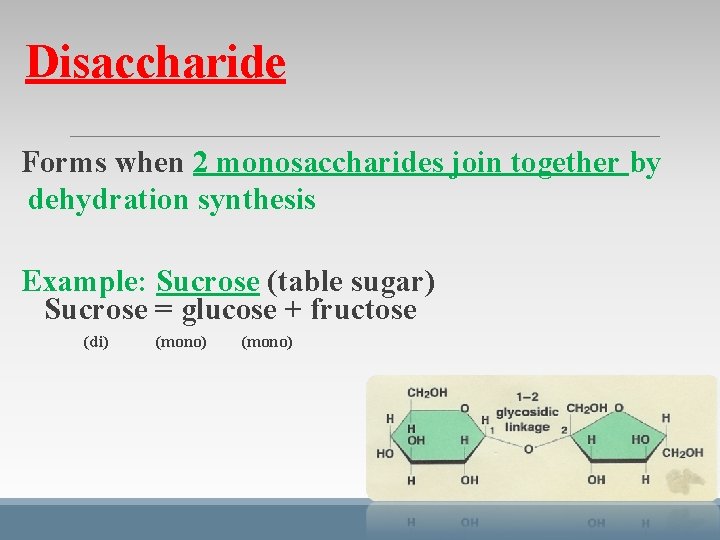
Disaccharide Forms when 2 monosaccharides join together by dehydration synthesis Example: Sucrose (table sugar) Sucrose = glucose + fructose (di) (mono)
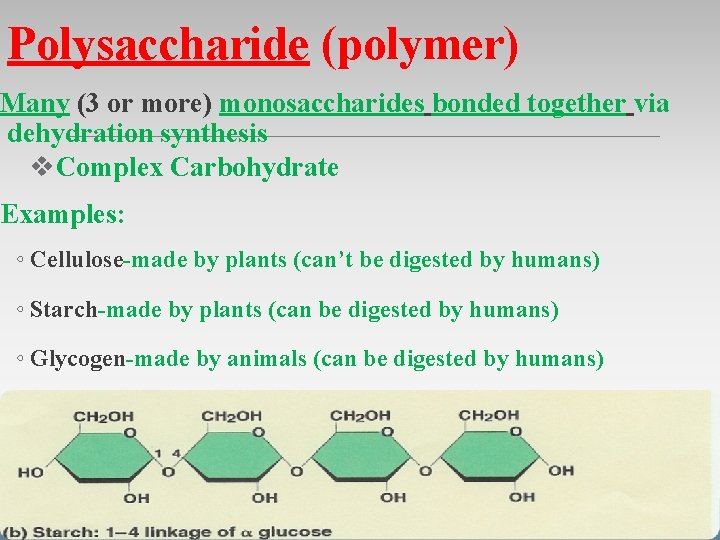
Polysaccharide (polymer) Many (3 or more) monosaccharides bonded together via dehydration synthesis v. Complex Carbohydrate Examples: ◦ Cellulose-made by plants (can’t be digested by humans) ◦ Starch-made by plants (can be digested by humans) ◦ Glycogen-made by animals (can be digested by humans)
Lipids (polymer) Function: Long-term energy storage Monomer: Fatty Acid Polymer: Lipid Contain Elements: C, H, O Description: Hydrocarbons that are insoluble (do not dissolve) in water/resistant to water.
Functions of Lipids Fats: triglycerides store energy (long term) Phospholipids: building blocks of cell membrane (lipid bi-layer) Steroids: like cholesterol, stabilize cell membranes, help create bile to digest fats, aide in the production of hormones Waxes: in plant cuticles to restrict water loss, in animal’s skin/hair to give protection and pliability, in bird feathers to waterproof Pigments: like chlorophyll that absorbs light during photosynthesis
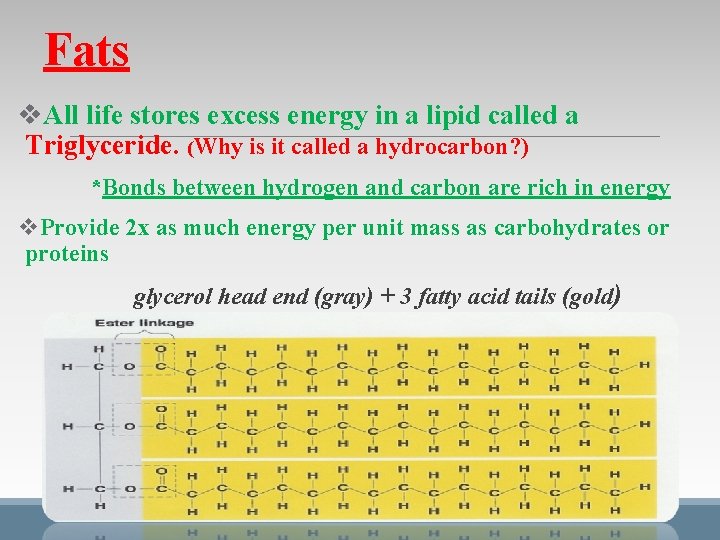
Fats v. All life stores excess energy in a lipid called a Triglyceride. (Why is it called a hydrocarbon? ) *Bonds between hydrogen and carbon are rich in energy v. Provide 2 x as much energy per unit mass as carbohydrates or proteins glycerol head end (gray) + 3 fatty acid tails (gold)
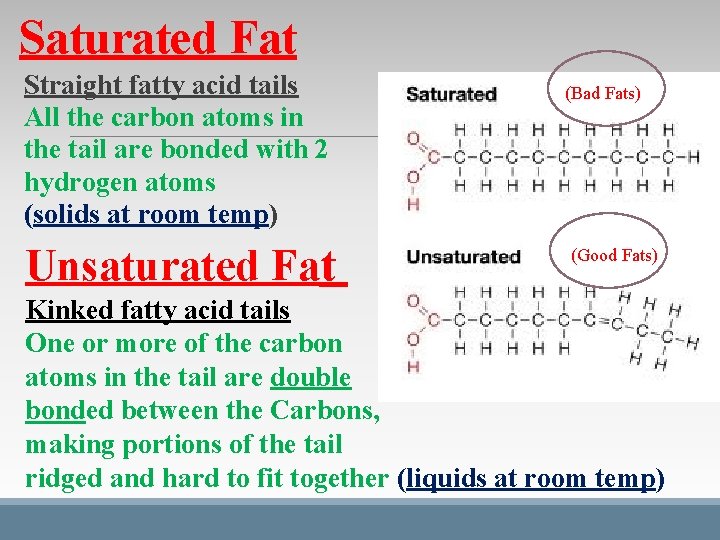
Saturated Fat Straight fatty acid tails All the carbon atoms in the tail are bonded with 2 hydrogen atoms (solids at room temp) Unsaturated Fat (Bad Fats) (Good Fats) Kinked fatty acid tails One or more of the carbon atoms in the tail are double bonded between the Carbons, making portions of the tail ridged and hard to fit together (liquids at room temp)
Good Fat vs. Bad Fat v THERE IS A HEALTH DIFFERENCE BETWEEN DIGESTED PLANT AND ANIMAL FATS SATURATED FAT – STRAIGHT TAILS/SOLIDS AT ROOM TEMP BAD FAT FOUND IN PRODUCTS DERIVED FROM ANIMALS. -BUTTER, LARD, & GREASE UNSATURATED FATS – KINKED TAILS/LIQUIDS AT ROOM TEMP GOOD FAT FOUND IN PRODUCTS DERIVED FROM PLANTS. -OLIVE OIL, FISH OIL, & PLANT OIL
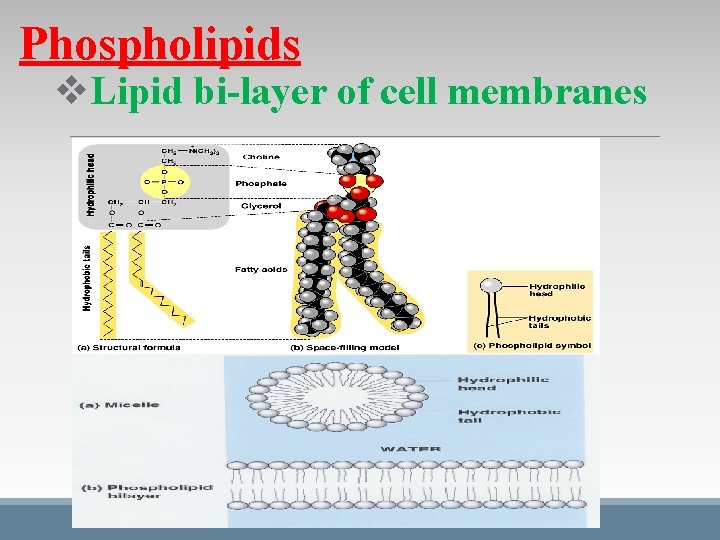
Phospholipids v. Lipid bi-layer of cell membranes
Proteins (polymer) Function: main building blocks of cells and involved in virtually all cell functions Monomer: Amino Acids Polymer: Protein v. Large molecule made of one or more chains of amino acids that are folded into a specific compact shape (shape determines function)
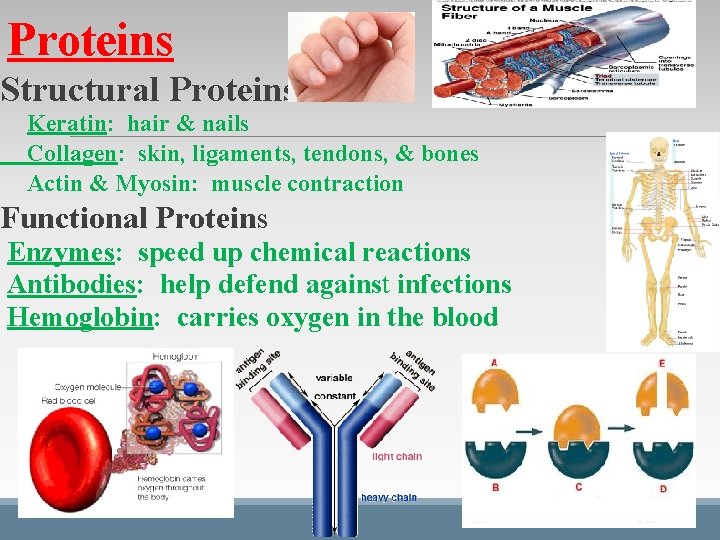
Proteins Structural Proteins Keratin: hair & nails Collagen: skin, ligaments, tendons, & bones Actin & Myosin: muscle contraction Functional Proteins Enzymes: speed up chemical reactions Antibodies: help defend against infections Hemoglobin: carries oxygen in the blood
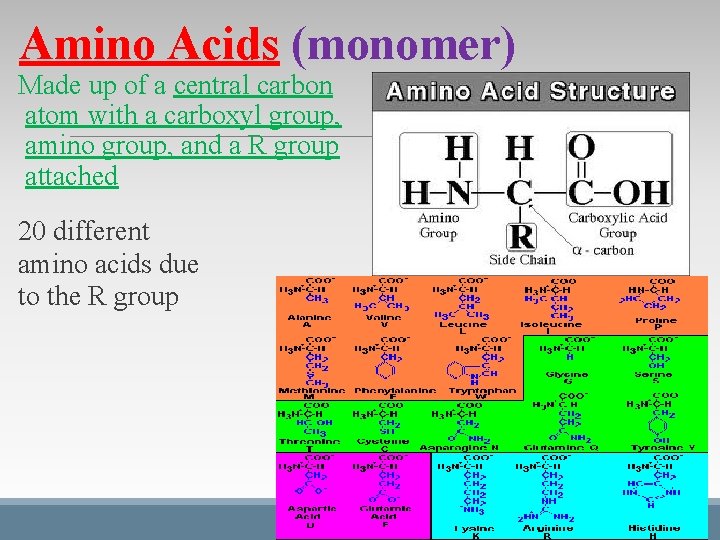
Amino Acids (monomer) Made up of a central carbon atom with a carboxyl group, amino group, and a R group attached 20 different amino acids due to the R group

Protein Sources 1 2 3
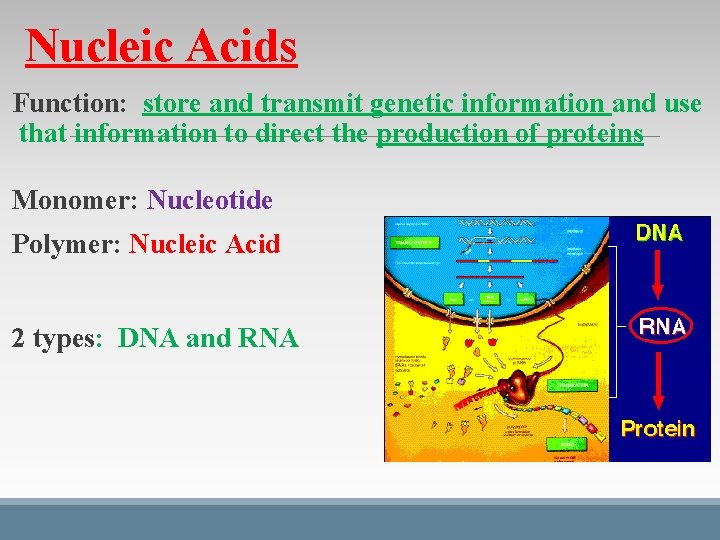
Nucleic Acids Function: store and transmit genetic information and use that information to direct the production of proteins Monomer: Nucleotide Polymer: Nucleic Acid 2 types: DNA and RNA
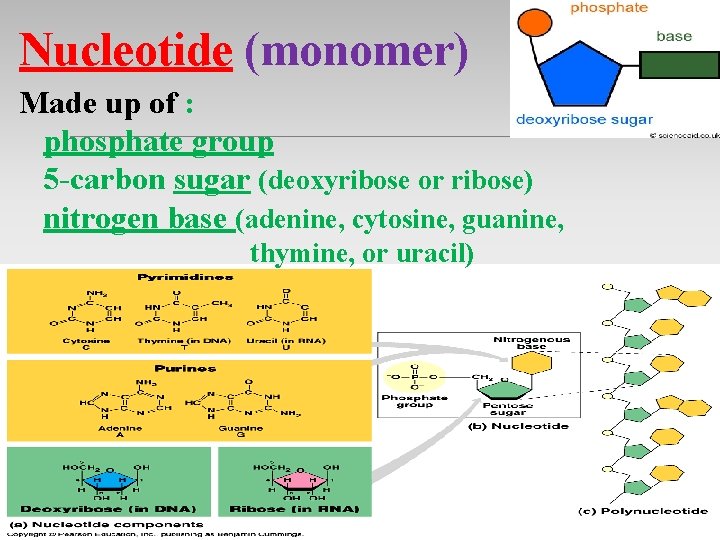
Nucleotide (monomer) Made up of : phosphate group 5 -carbon sugar (deoxyribose or ribose) nitrogen base (adenine, cytosine, guanine, thymine, or uracil)
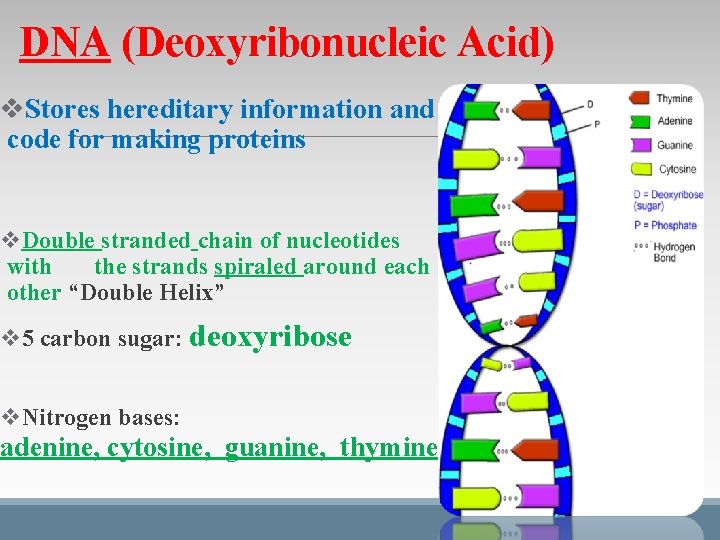
DNA (Deoxyribonucleic Acid) v. Stores hereditary information and code for making proteins v. Double stranded chain of nucleotides with the strands spiraled around each other “Double Helix” v 5 carbon sugar: deoxyribose v. Nitrogen bases: adenine, cytosine, guanine, thymine
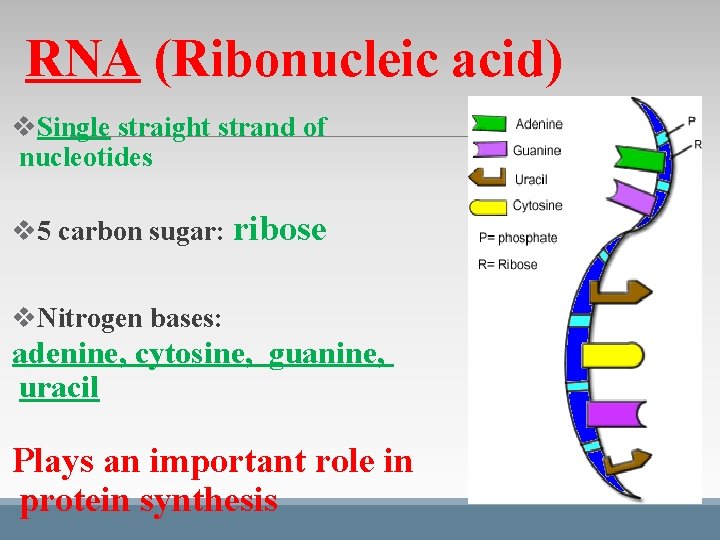
RNA (Ribonucleic acid) v. Single straight strand of nucleotides v 5 carbon sugar: ribose v. Nitrogen bases: adenine, cytosine, guanine, uracil Plays an important role in protein synthesis
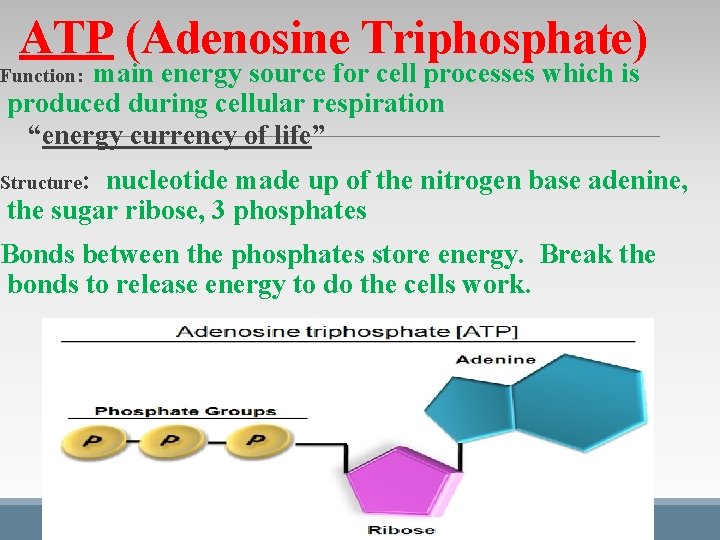
ATP (Adenosine Triphosphate) main energy source for cell processes which is produced during cellular respiration “energy currency of life” Function: Structure: nucleotide made up of the nitrogen base adenine, the sugar ribose, 3 phosphates Bonds between the phosphates store energy. Break the bonds to release energy to do the cells work.
- Slides: 32