Biomolecules The Building Blocks of Life Biomolecules are
Biomolecules The Building Blocks of Life
Biomolecules are Organic Molecules- contains Carbon bonds 1. Molecules containing Carbon, Hydrogen, Nitrogen, and often Oxygen. 2. They make up living organisms 3. Examples: Methane (CH 4) Glucose (C 6 H 12 O 6) are all organic molecules
Biomolecules 1. Basic Molecule: Proteins, Carbohydrates (sugars and starch), Lipids (Fats), Nucleic Acid (DNA, RNA) 2. Macromolecule: Large molecules of the above that can be broken down. • Ex. Starch made of many sugars
Biomolecules 1. Subunits: The smaller molecules that are the building blocks of macro molecules • Sugars that make up starch or cellulose • Amino Acids that make up Proteins • Fatty acids and glycerol make up lipids
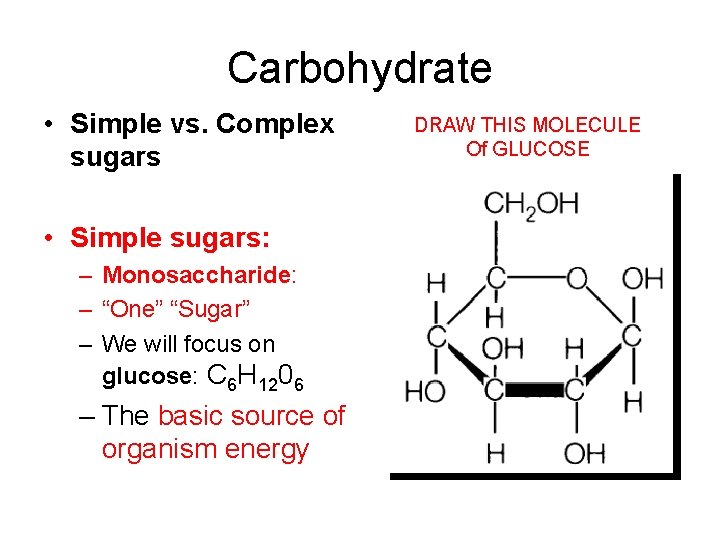
Carbohydrate • Simple vs. Complex sugars • Simple sugars: – Monosaccharide: – “One” “Sugar” – We will focus on glucose: C 6 H 1206 – The basic source of organism energy DRAW THIS MOLECULE Of GLUCOSE
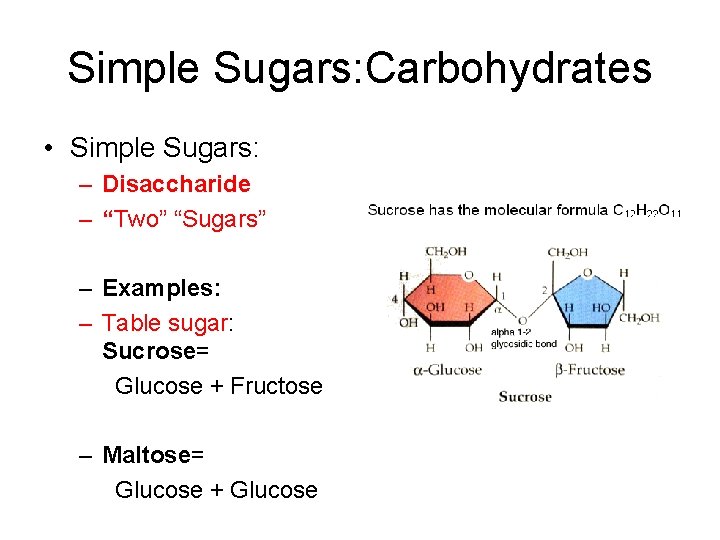
Simple Sugars: Carbohydrates • Simple Sugars: – Disaccharide – “Two” “Sugars” – Examples: – Table sugar: Sucrose= Glucose + Fructose – Maltose= Glucose + Glucose
Complex Sugars: Polysaccharide • Poly “many”, Saccharide “sugars” • Functions: Cells use them for energy and structure. • They allow organisms to gradually use energy since it is stored in a large structure. (like money in a bank)
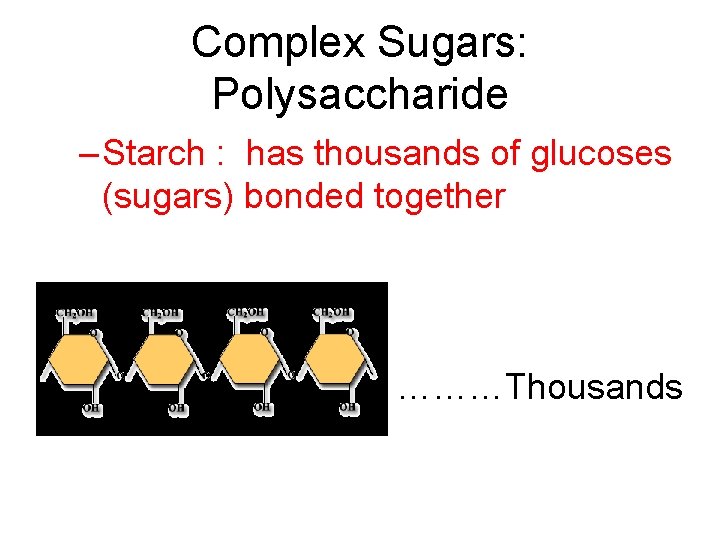
Complex Sugars: Polysaccharide – Starch : has thousands of glucoses (sugars) bonded together ………Thousands

Complex Sugars: Polysaccharides • Cellulose: Makes up the walls of plant cells. Also made from glucose. • Ruminants (cattle, sheep) can digest both cellulose and glucose. • Humans can digest starch, but not cellulose WHY? ? ? ? ?
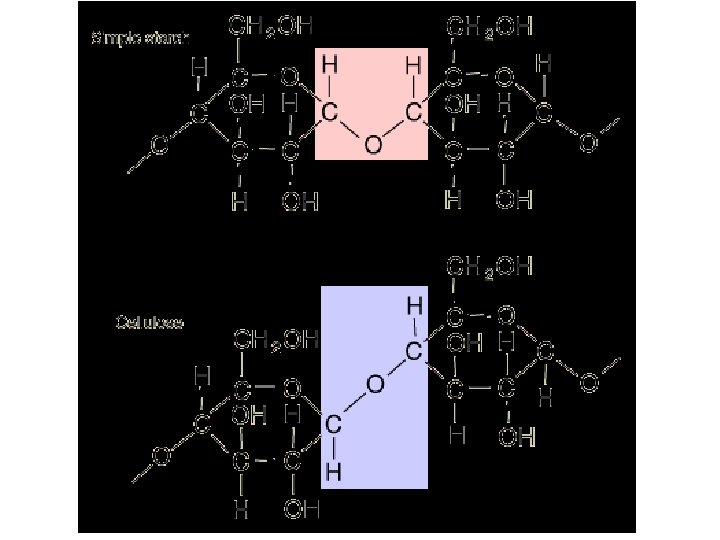
Polysaccharides • Glycogen: Animals store carbohydrates (glucose) in the form of glycogen; similar in form to starch. Why? ? • This is why… – This is our reserve energy – Stored in liver and muscles – We do not want to lose our carbs all at once!!
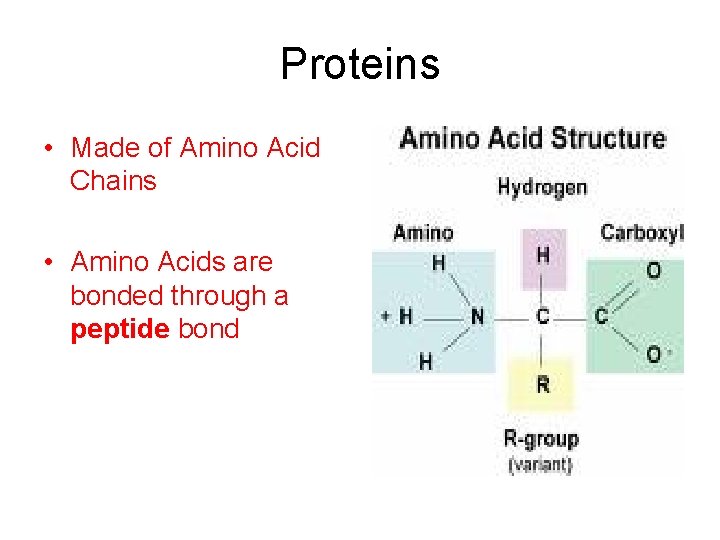
Proteins • Made of Amino Acid Chains • Amino Acids are bonded through a peptide bond
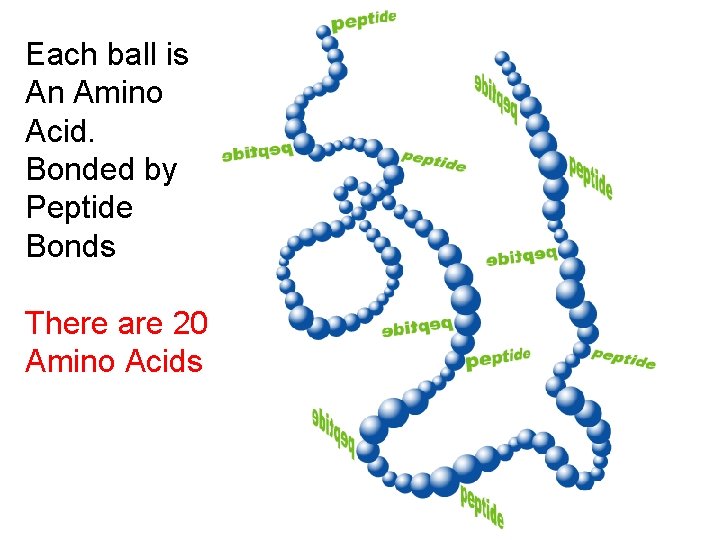
Each ball is An Amino Acid. Bonded by Peptide Bonds There are 20 Amino Acids
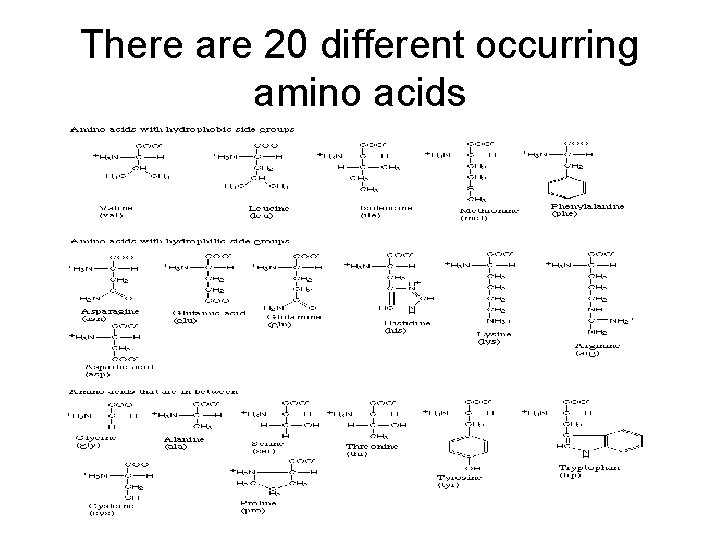
There are 20 different occurring amino acids
Protein Function 1. Building material: muscle, hair, fingernails 2. Enzymes: Help with chemical reaction in the cells and body (catalyst) 3. Immunity: make up antibodies 4. Other specific functions such as Hemoglobin: carry O 2 in red blood cells
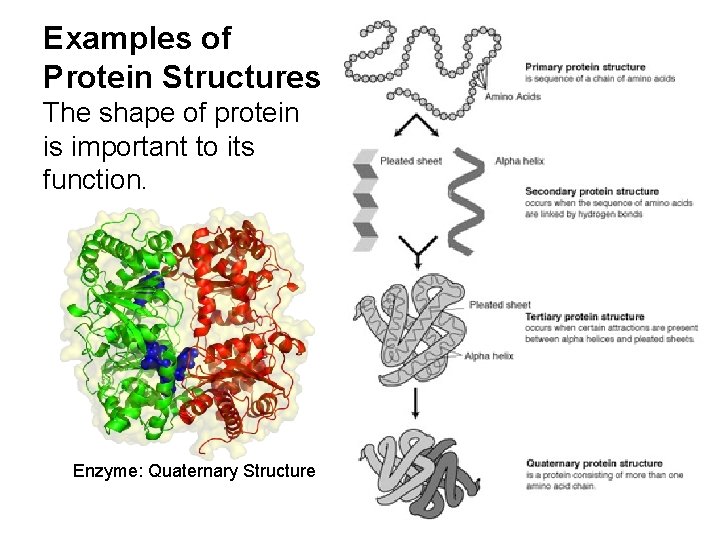
Examples of Protein Structures The shape of protein is important to its function. Enzyme: Quaternary Structure
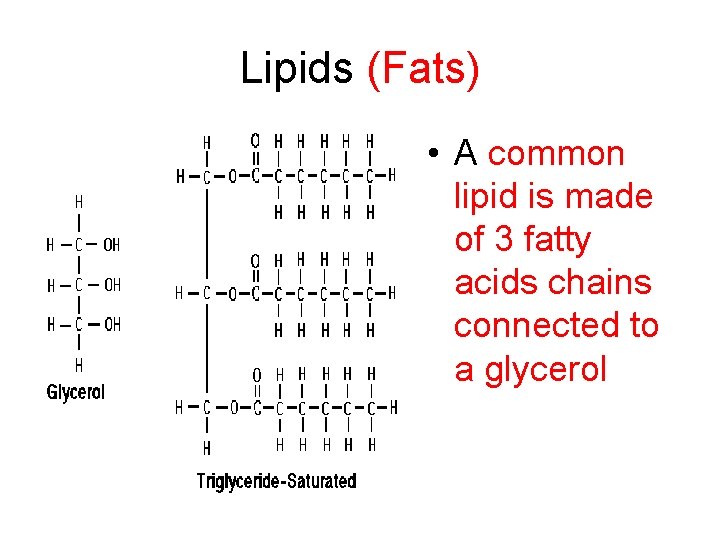
Lipids (Fats) • A common lipid is made of 3 fatty acids chains connected to a glycerol

Lipids (Fats) • Glycerol: a type of alcohol. The back bone of Fats. • 3 Fatty acid chains: Long chains of C & H – Saturated=as many C & H bonded as possible (Solid at Room Temp. ) ex. Lard!! – Unsaturated= C and C bonds can be double (usually Liquid at Room Temp. ) ex. Corn oil
Lipids (Fats) Functions • The main energy storing molecule because of the high # of carbon to carbon bonds. Why are bonds important? • …because they Store chemical energy • Lipids store more energy than any other biomolecule – 9 Cal/gram = lipids – 4 Cal/gram = carbohydrates and proteins
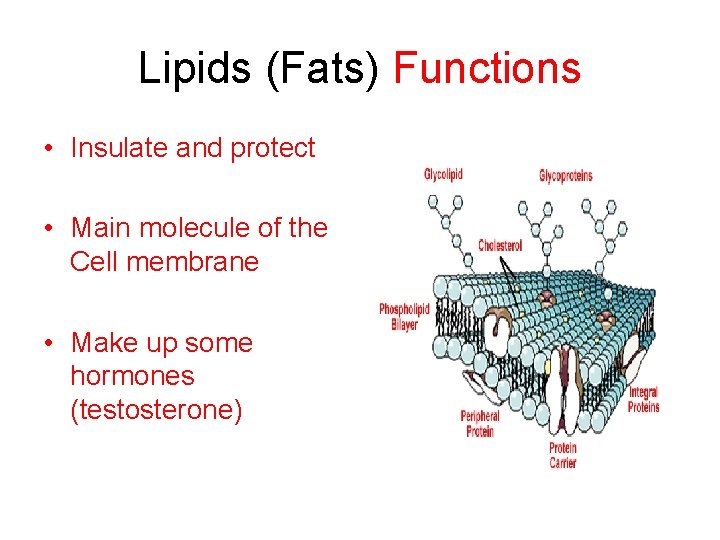
Lipids (Fats) Functions • Insulate and protect • Main molecule of the Cell membrane • Make up some hormones (testosterone)
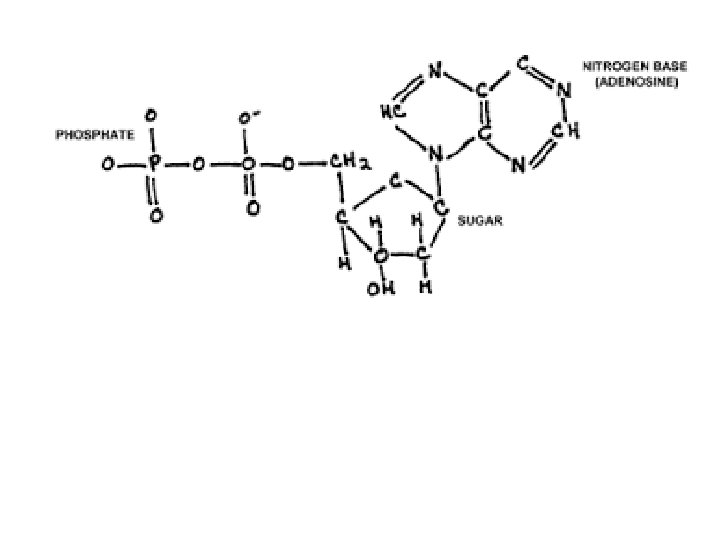
Nucleic Acids • Large organic molecules that determine the genetic traits of an organism. • Small units of Nucleic Acids are called nucleotides which are made of carbon, hydrogen, oxygen and nitrogen • When nucleotides bond with sugar and phosphates, they form DNA and RNA

? Questions? • What type of bond connects Amino Acids? • What are lipids composed of? • What are three of the macromolecules of carbohydrates? What is the sugar subunit? • What are the four types of elements that make up biomolecules?
- Slides: 23