Biomolecules Carbohydrates Proteins Lipids and Nucleic acids Carbon
Biomolecules Carbohydrates Proteins, Lipids, and Nucleic acids

Carbon is the central element • All biomolecules contain a Carbon chain or ring • Carbon has 4 outer shell electrons (valence = 4) • Therefore it’s bonding capacity is great • It forms covalent bonds –hence, has strong bonds • Once bound to other elements (or to other Carbons), it is very stable

Carbon linkages • Single chains • Rings Propane
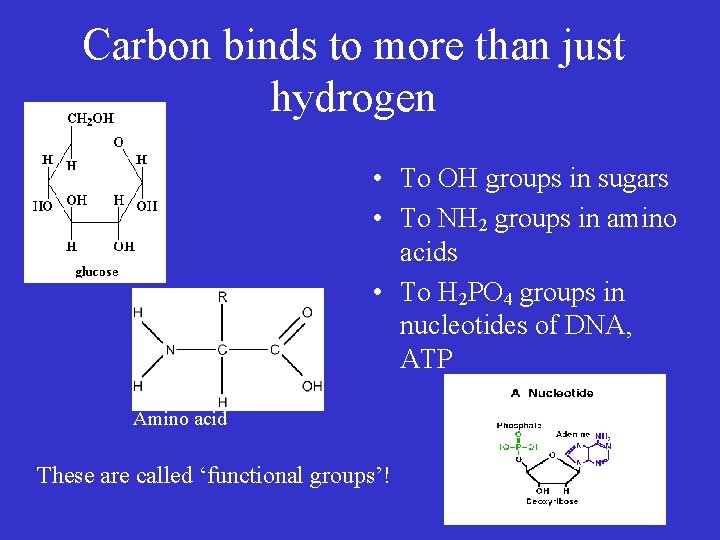
Carbon binds to more than just hydrogen • To OH groups in sugars • To NH 2 groups in amino acids • To H 2 PO 4 groups in nucleotides of DNA, ATP Amino acid These are called ‘functional groups’!

Carbohydrates (or sugars) • Simple sugars (monosaccharides) • Only one 6 -C chain or ring involved • General formula (CH 2 O)

Carbohydrates (sugars) Lactose • Double sugars (disaccharides) • Two 6 -C chains or rings bonded together
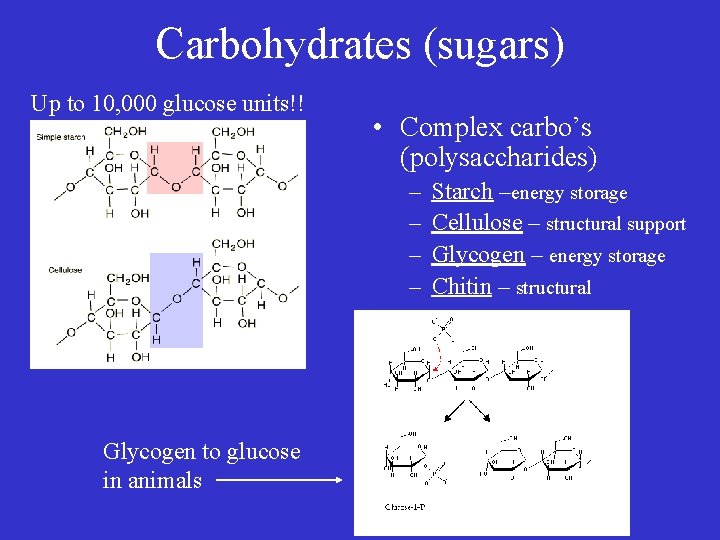
Carbohydrates (sugars) Up to 10, 000 glucose units!! • Complex carbo’s (polysaccharides) – – Glycogen to glucose in animals Starch –energy storage Cellulose – structural support Glycogen – energy storage Chitin – structural
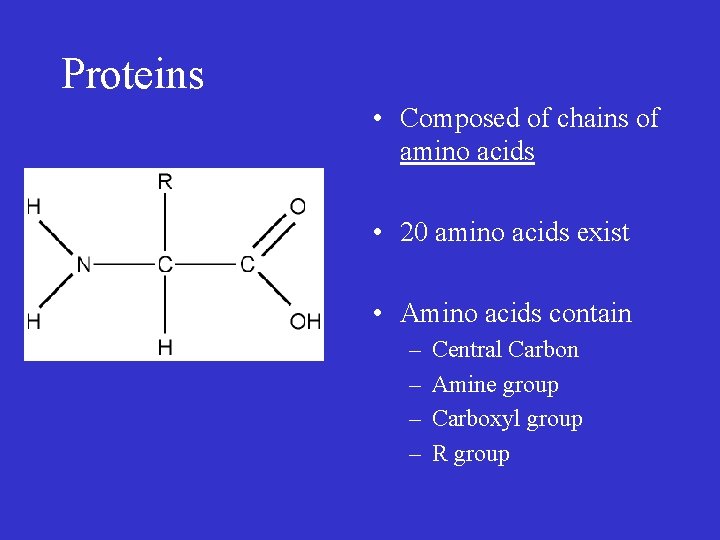
Proteins • Composed of chains of amino acids • 20 amino acids exist • Amino acids contain – – Central Carbon Amine group Carboxyl group R group
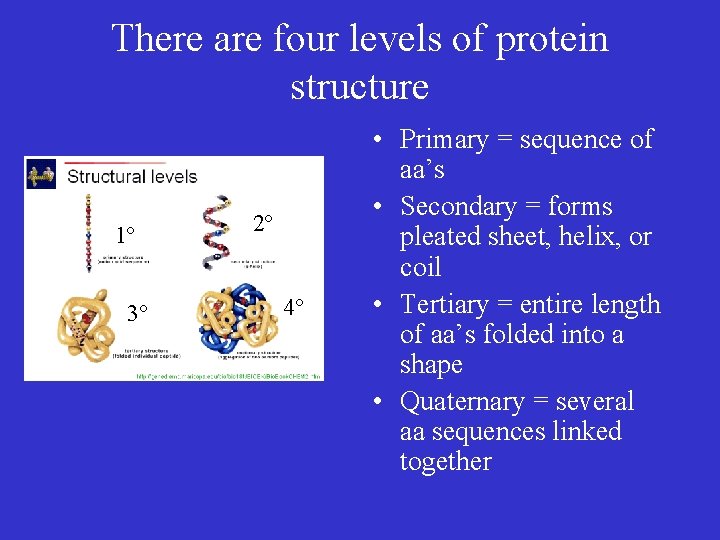
There are four levels of protein structure 1º 3° 2º 4° • Primary = sequence of aa’s • Secondary = forms pleated sheet, helix, or coil • Tertiary = entire length of aa’s folded into a shape • Quaternary = several aa sequences linked together
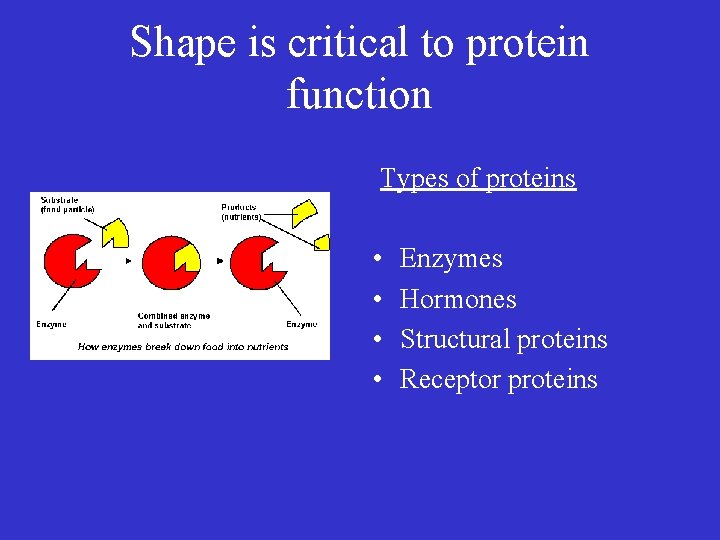
Shape is critical to protein function Types of proteins • • Enzymes Hormones Structural proteins Receptor proteins
Nucleic acids: DNA and RNA • DNA = deoxyribonucleic acid • DNA is a double polymer (chain) • Each chain is made of nucleotides • The 2 chains bond together to form a helix
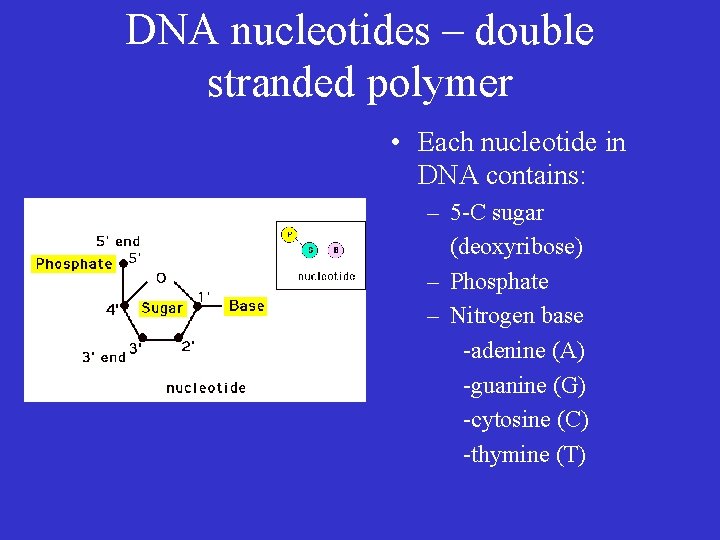
DNA nucleotides – double stranded polymer • Each nucleotide in DNA contains: – 5 -C sugar (deoxyribose) – Phosphate – Nitrogen base -adenine (A) -guanine (G) -cytosine (C) -thymine (T)
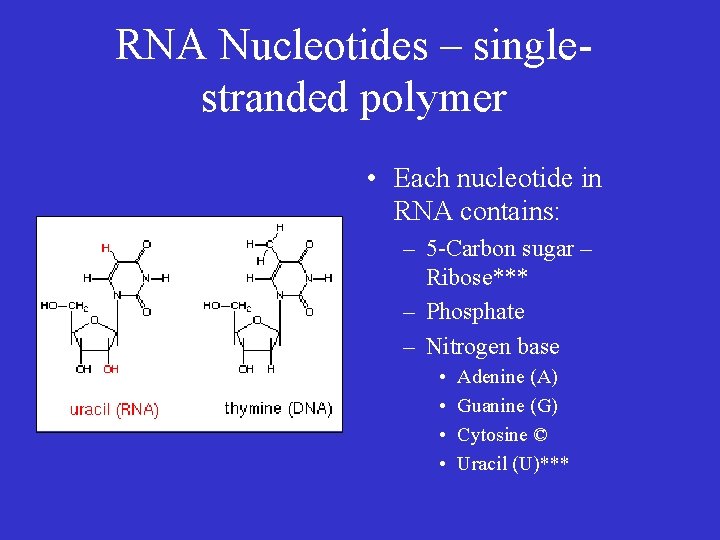
RNA Nucleotides – singlestranded polymer • Each nucleotide in RNA contains: – 5 -Carbon sugar – Ribose*** – Phosphate – Nitrogen base • • Adenine (A) Guanine (G) Cytosine © Uracil (U)***

Lipids • Made of the same elements as carbohydrates, but insoluble in water • Do not form polymers! • Major function: – Energy storage – Insulation – Cell membranes
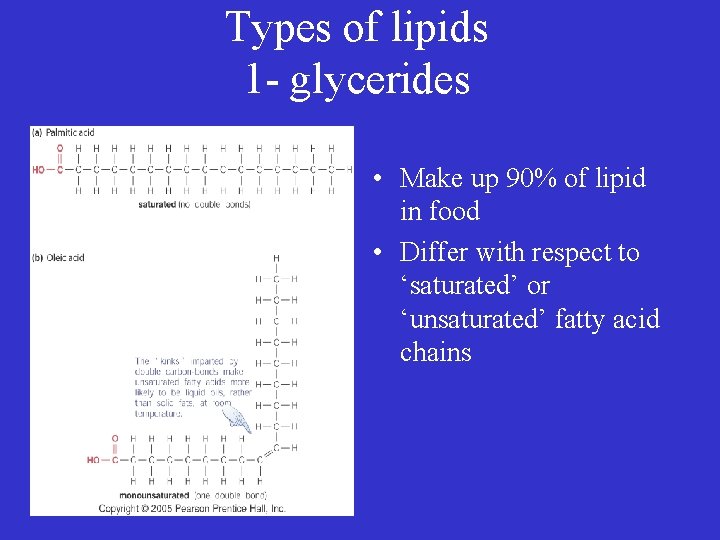
Types of lipids 1 - glycerides • Make up 90% of lipid in food • Differ with respect to ‘saturated’ or ‘unsaturated’ fatty acid chains
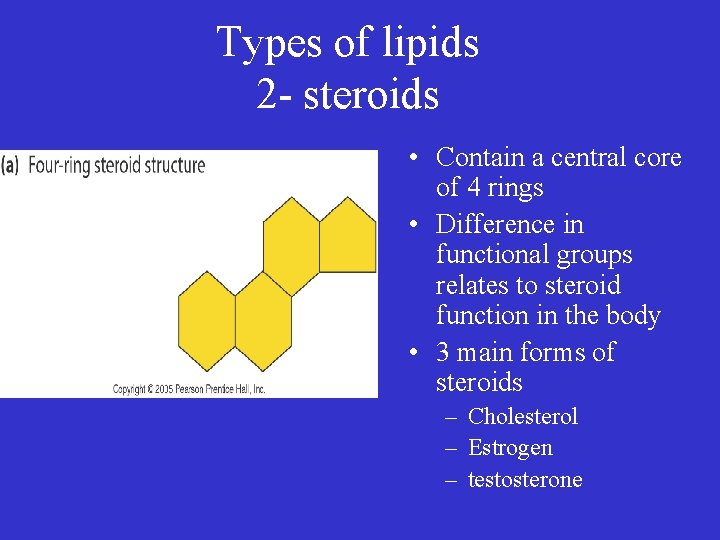
Types of lipids 2 - steroids • Contain a central core of 4 rings • Difference in functional groups relates to steroid function in the body • 3 main forms of steroids – Cholesterol – Estrogen – testosterone

Lipids and Health • Hierarchy of dietary fats: – Fats containing Omega -3 fatty acids – Mono- and poly unsaturated fats – Saturated fats – Trans fats
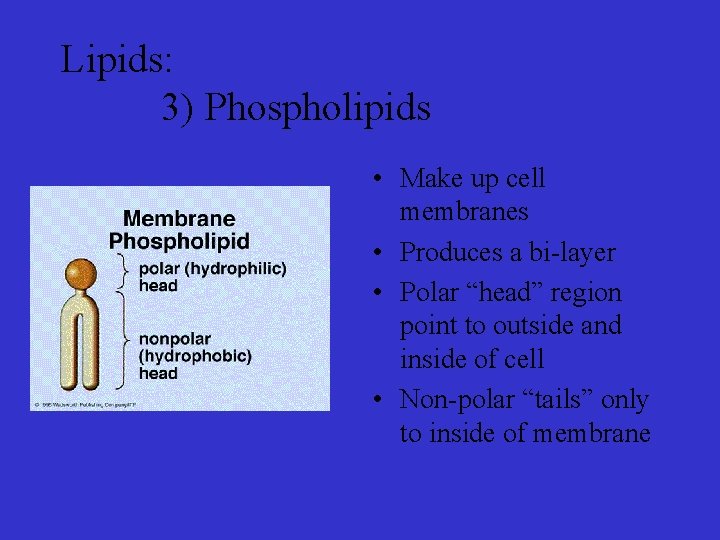
Lipids: 3) Phospholipids • Make up cell membranes • Produces a bi-layer • Polar “head” region point to outside and inside of cell • Non-polar “tails” only to inside of membrane
- Slides: 18