Biomolecules and the Chemistry of Life Ruth Colyer
Biomolecules and the Chemistry of Life Ruth Colyer Juarez-Lincoln High School
p. H and Buffers The concentration of hydrogen ions in a solution is very important for living things. This is because the hydrogen ions are positively charged and can alter the charge of the environment of other molecules in solution. By putting different forces on the molecules, the molecules change shape from their normal shape.
![p. H and Buffers The concentration of hydrogen ions, [H+], is commonly expressed in p. H and Buffers The concentration of hydrogen ions, [H+], is commonly expressed in](http://slidetodoc.com/presentation_image_h2/a9f033f400ba825a42f7fd2d25feb11b/image-3.jpg)
p. H and Buffers The concentration of hydrogen ions, [H+], is commonly expressed in terms of p. H, where p. H = -log[H+]. ↓p. H = ↑[H+] and ↑p. H = ↓[H+] A substance that increases the [H+] (lowers the p. H) is called an acid. A substance that reduces the [H+] (raises the p. H) is called a base.
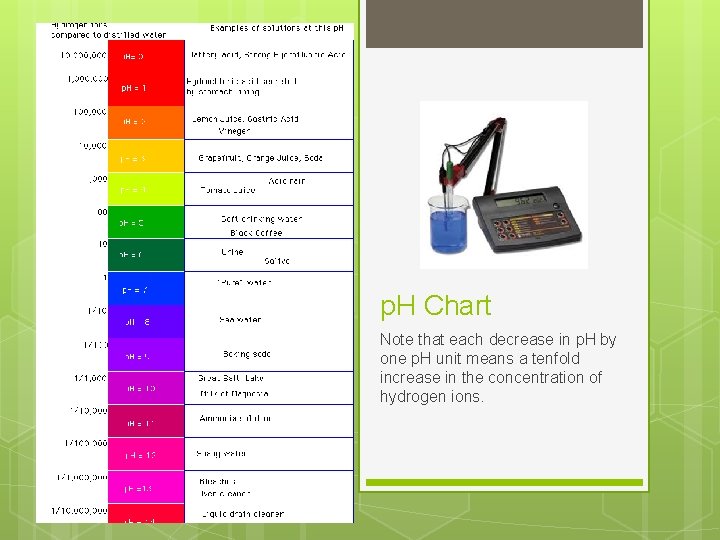
p. H Chart Note that each decrease in p. H by one p. H unit means a tenfold increase in the concentration of hydrogen ions.
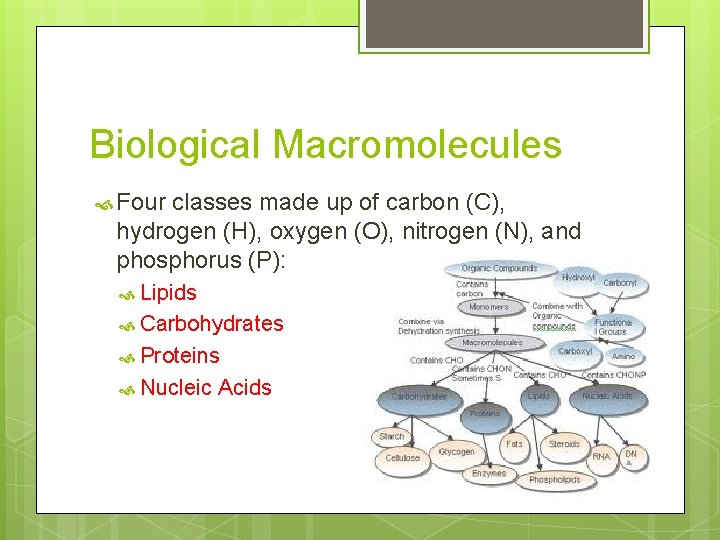
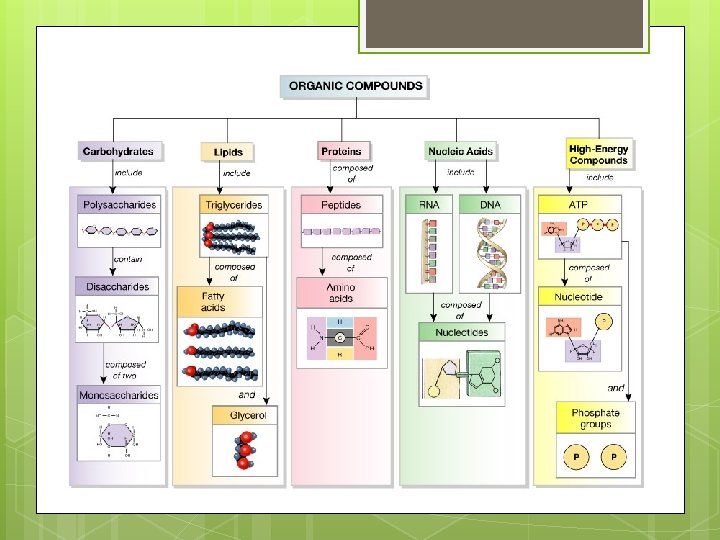
Biological Macromolecules Four classes made up of carbon (C), hydrogen (H), oxygen (O), nitrogen (N), and phosphorus (P): Lipids Carbohydrates Proteins Nucleic Acids
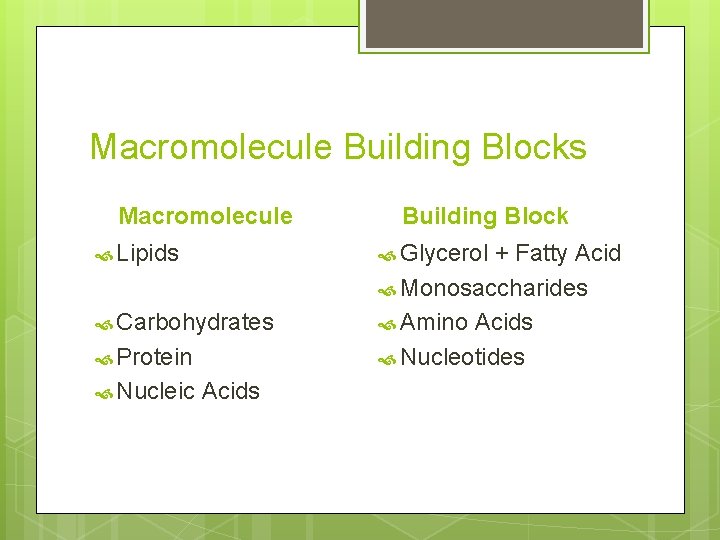
Macromolecule Building Blocks Macromolecule Lipids Glycerol Carbohydrates Protein Nucleic Building Block Acids + Fatty Acid Monosaccharides Amino Acids Nucleotides
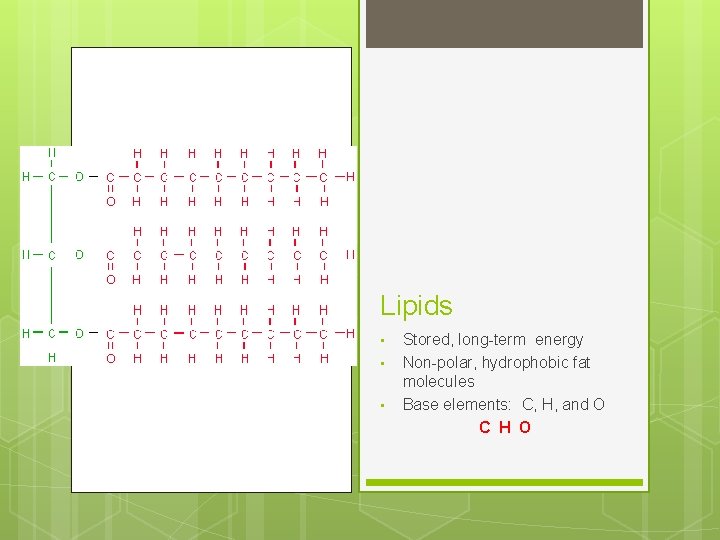
Lipids • • • Stored, long-term energy Non-polar, hydrophobic fat molecules Base elements: C, H, and O C H O
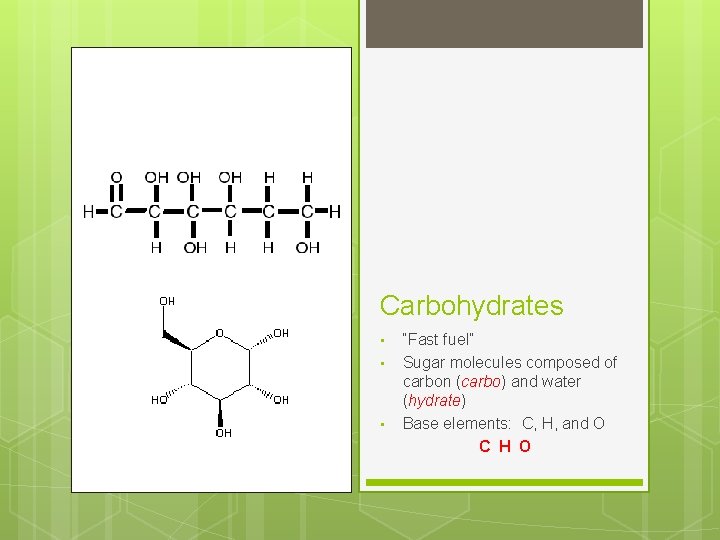
Carbohydrates • • • “Fast fuel” Sugar molecules composed of carbon (carbo) and water (hydrate) Base elements: C, H, and O C H O
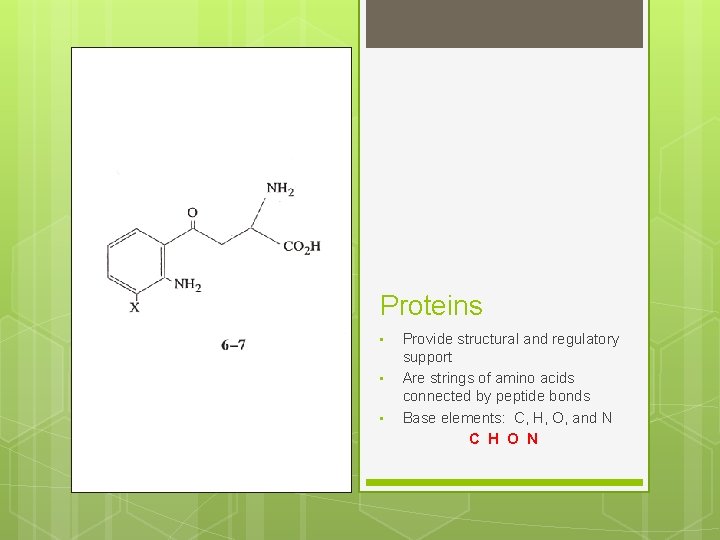
Proteins • • • Provide structural and regulatory support Are strings of amino acids connected by peptide bonds Base elements: C, H, O, and N C H O N
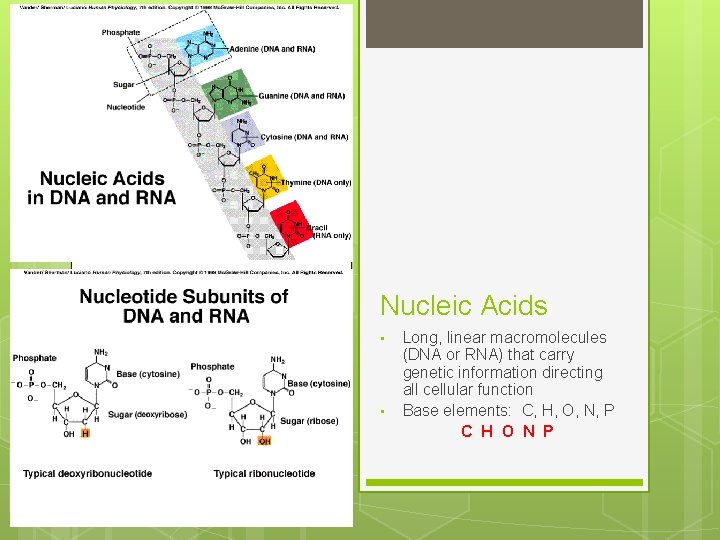
Nucleic Acids • • Long, linear macromolecules (DNA or RNA) that carry genetic information directing all cellular function Base elements: C, H, O, N, P C H O N P
- Slides: 11