Biology Basic Chemistry Matter and Atomic Structure o
Biology Basic Chemistry
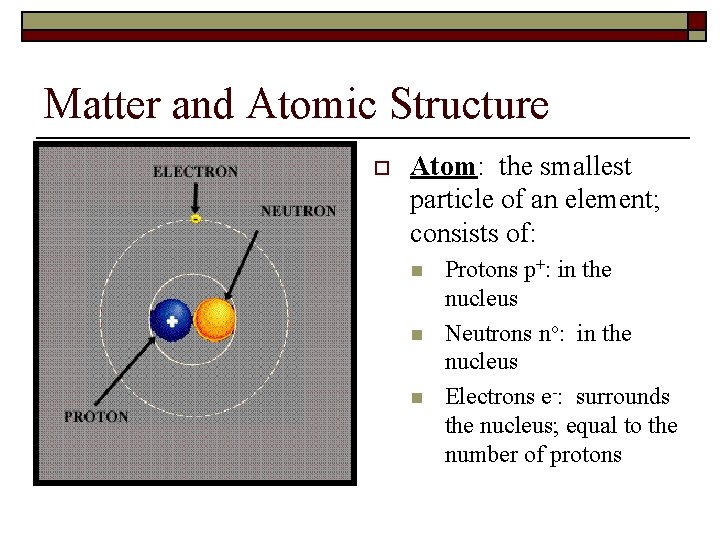
Matter and Atomic Structure o Atom: the smallest particle of an element; consists of: n n n Protons p+: in the nucleus Neutrons no: in the nucleus Electrons e-: surrounds the nucleus; equal to the number of protons
Matter and Atomic Structure o o Matter: anything that has volume and mass Element: a substance not broken down into simpler substances by physical or chemical means n Each element has a 1 or 2 -letter symbol o o Molecule: A molecule is formed when two or more atoms join together chemically. n o Examples: oxygen (O), sodium (Na) Ex: hydrogen (H 2), oxygen (O 2) and nitrogen (N 2) Compound: a molecule composed of atoms of 2+ different elements that are chemically combined n Ex: Na. Cl: salt, H 2 O: water
Matter and Atomic Structure o o Atomic Number: the number of protons in an atom’s nucleus Mass Number: the number of protons and neutrons in an atom Energy levels: the area of an atom surrounding the nucleus where electrons are found # of protons always equals the # of electrons; atoms have NO CHARGE
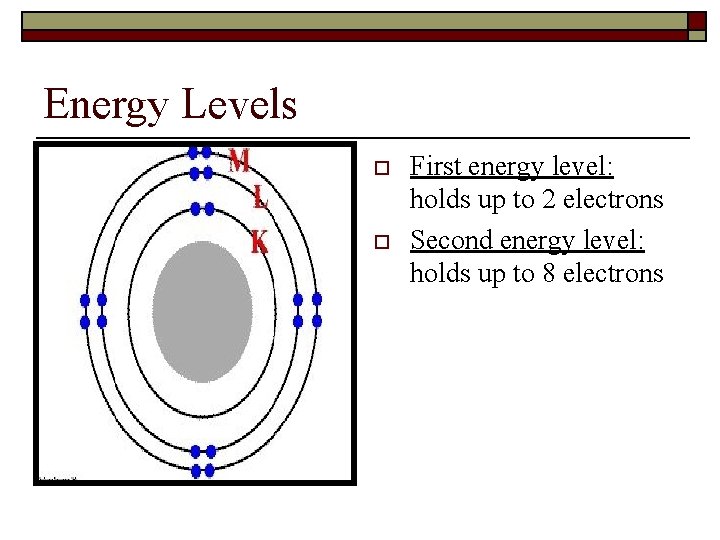
Energy Levels o o First energy level: holds up to 2 electrons Second energy level: holds up to 8 electrons

Chemical Bonds The main types of chemical bonds are: o ionic bonds o covalent bonds Copyright Pearson Prentice Hall
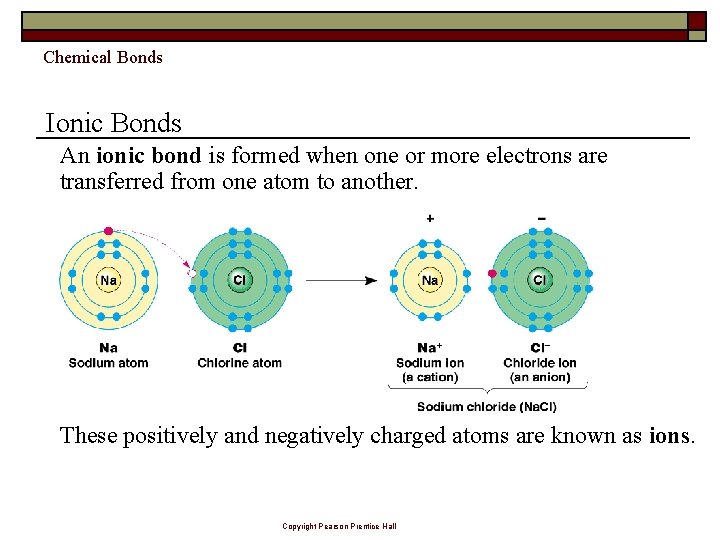
Chemical Bonds Ionic Bonds An ionic bond is formed when one or more electrons are transferred from one atom to another. These positively and negatively charged atoms are known as ions. Copyright Pearson Prentice Hall
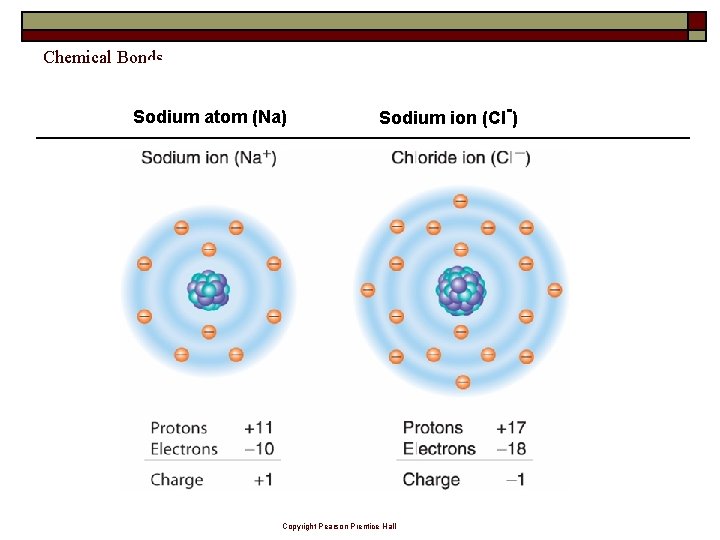
Chemical Bonds Sodium atom (Na) Sodium ion (Cl-) Copyright Pearson Prentice Hall
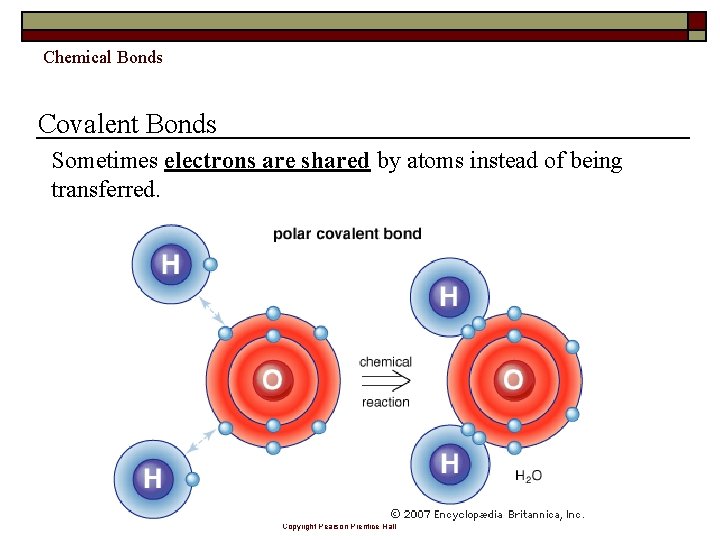
Chemical Bonds Covalent Bonds Sometimes electrons are shared by atoms instead of being transferred. Copyright Pearson Prentice Hall
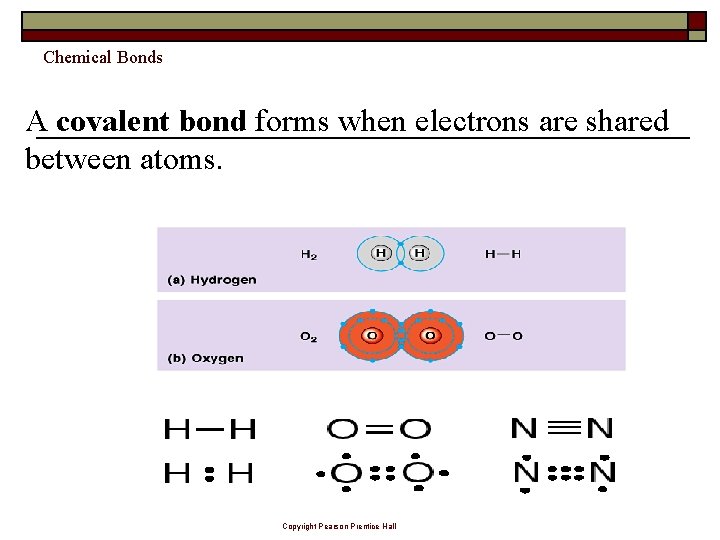
Chemical Bonds A covalent bond forms when electrons are shared between atoms. Copyright Pearson Prentice Hall
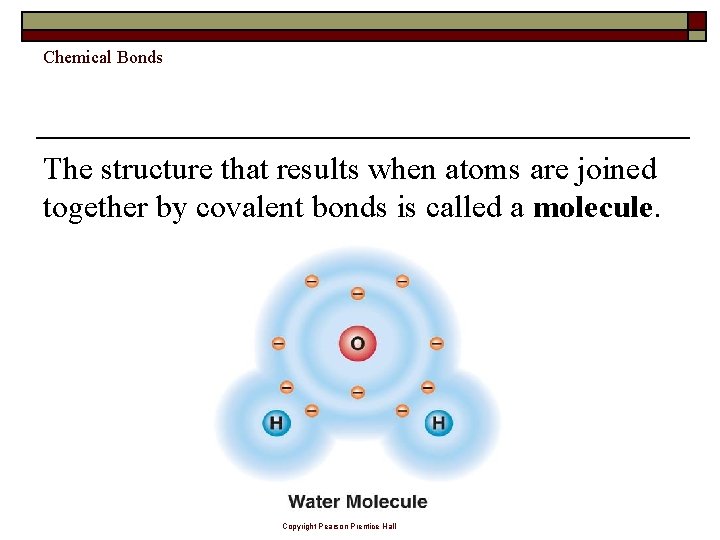
Chemical Bonds The structure that results when atoms are joined together by covalent bonds is called a molecule. Copyright Pearson Prentice Hall

Water o About 60 -90 percent of an organism is water Water is used in most reactions in the body Water is called the universal solvent
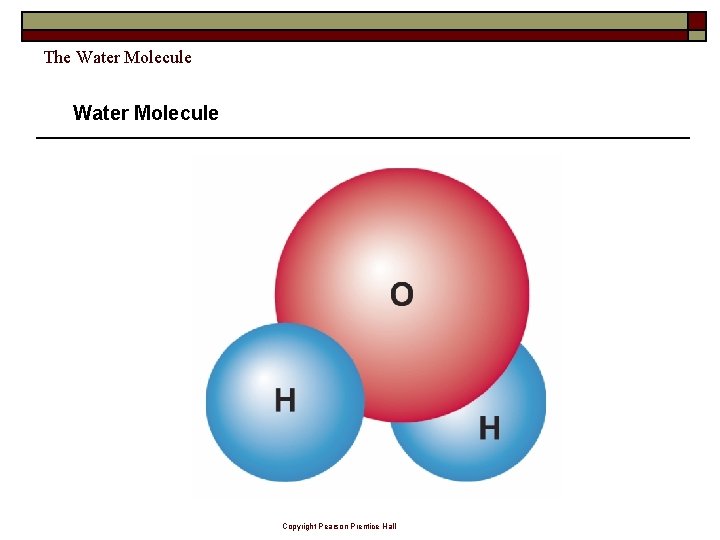
The Water Molecule Copyright Pearson Prentice Hall
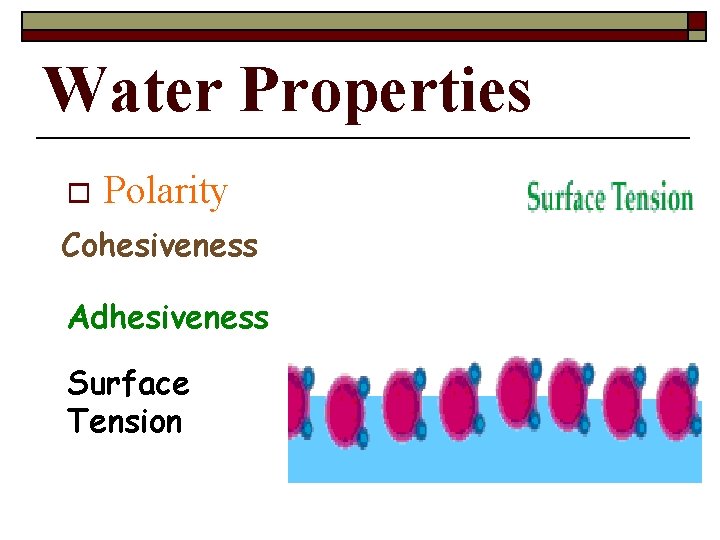
Water Properties o Polarity Cohesiveness Adhesiveness Surface Tension
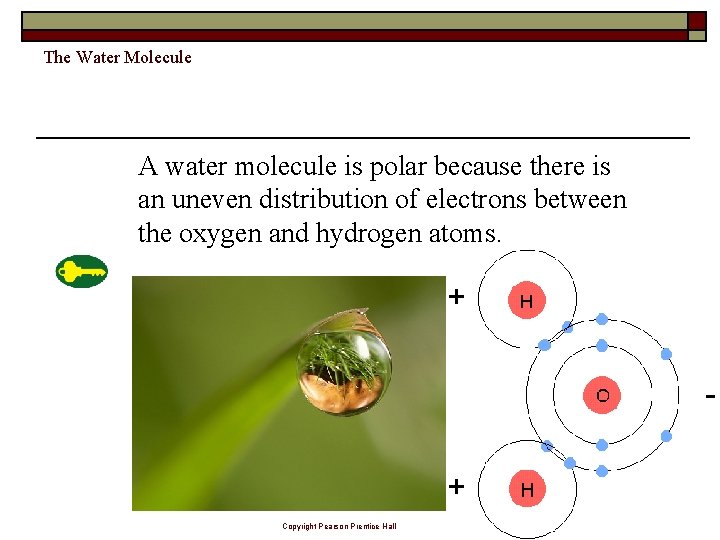
The Water Molecule A water molecule is polar because there is an uneven distribution of electrons between the oxygen and hydrogen atoms. Copyright Pearson Prentice Hall
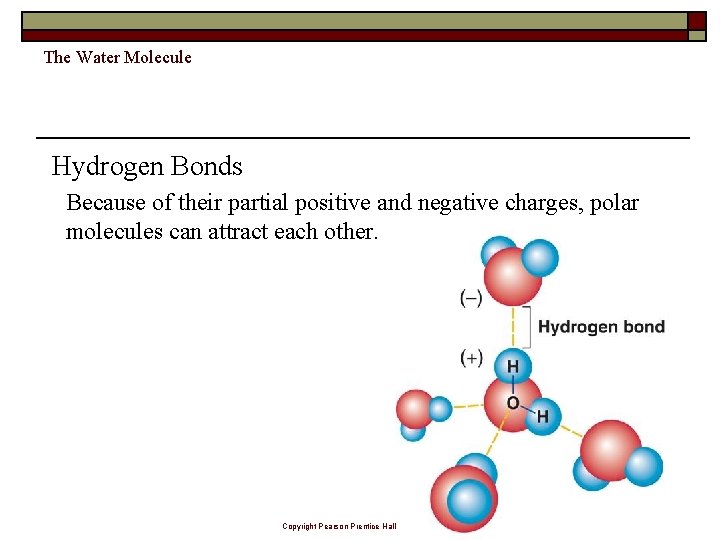
The Water Molecule Hydrogen Bonds Because of their partial positive and negative charges, polar molecules can attract each other. Copyright Pearson Prentice Hall

The Water Molecule Cohesion is an attraction between molecules of the same substance. Because of hydrogen bonding, water is extremely cohesive. n. Example: surface tension (bugs walking on water) Copyright Pearson Prentice Hall
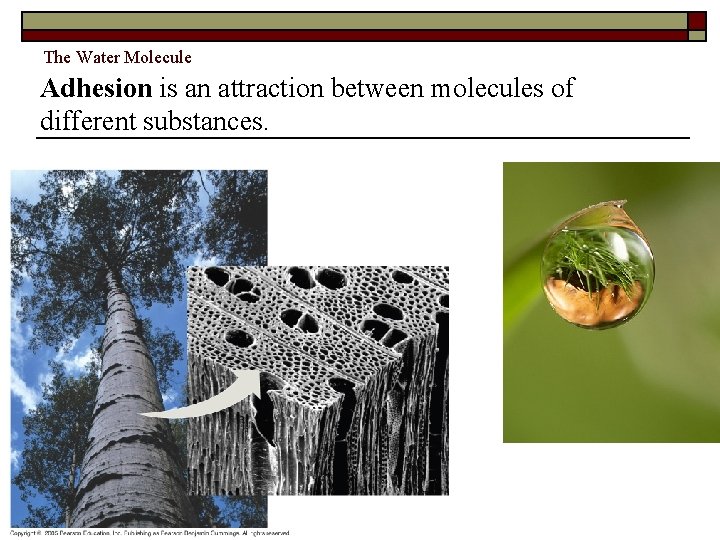
The Water Molecule Adhesion is an attraction between molecules of different substances. Copyright Pearson Prentice Hall

Solutions and Suspensions A mixture is a material composed of two or more elements or compounds that are physically mixed but not chemically combined. Copyright Pearson Prentice Hall

Solutions and Suspensions Two types of mixtures can be made with water n solutions n suspensions Copyright Pearson Prentice Hall

Solutions and Suspensions Solutions All the components of a solution are evenly distributed throughout the solution. solute—the substance that is dissolved. solvent—the substance in which the solute dissolves. Copyright Pearson Prentice Hall
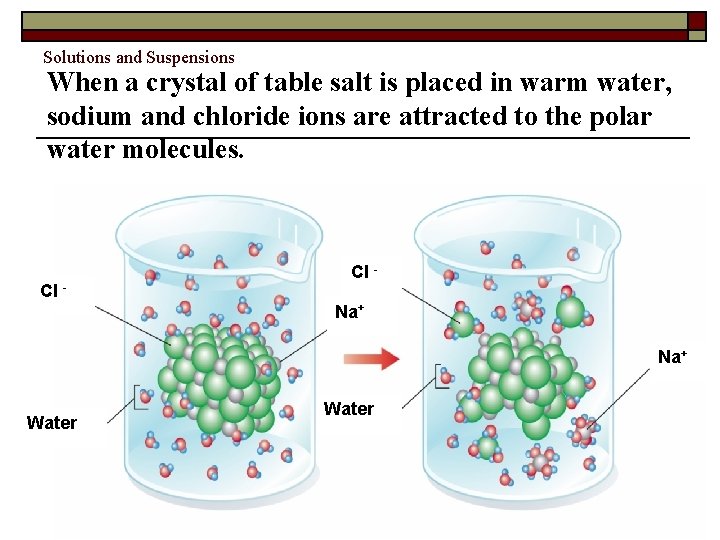
Solutions and Suspensions When a crystal of table salt is placed in warm water, sodium and chloride ions are attracted to the polar water molecules. Cl - Cl Na+ Water Copyright Pearson Prentice Hall
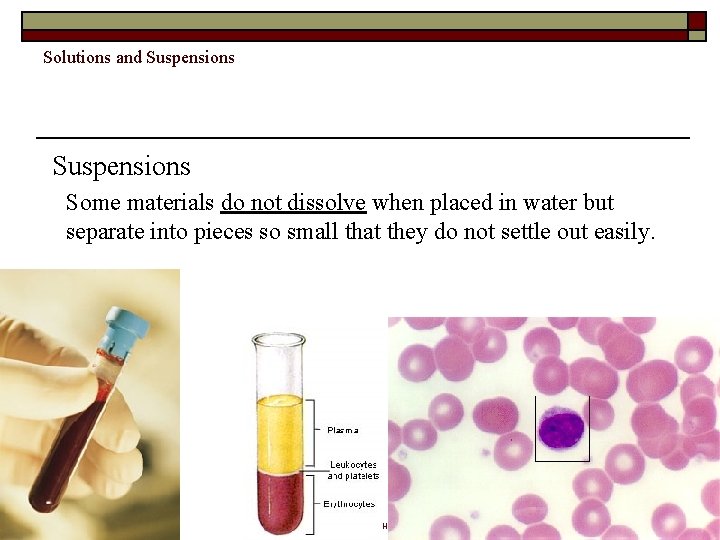
Solutions and Suspensions Some materials do not dissolve when placed in water but separate into pieces so small that they do not settle out easily. Copyright Pearson Prentice Hall
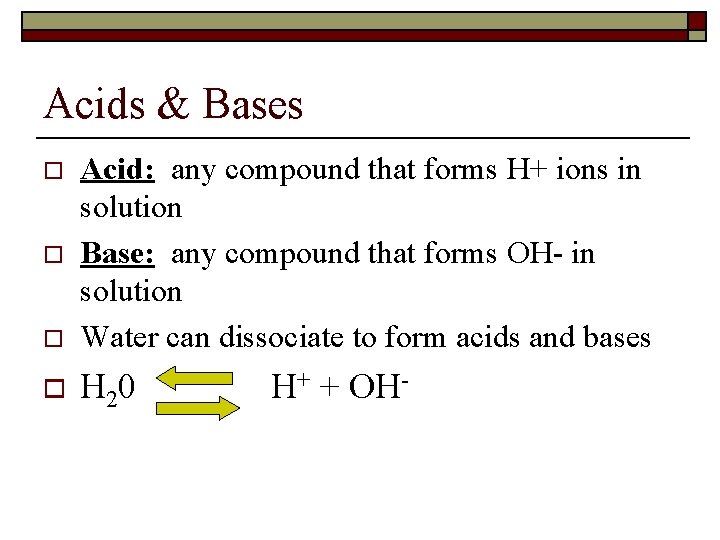
Acids & Bases o Acid: any compound that forms H+ ions in solution Base: any compound that forms OH- in solution Water can dissociate to form acids and bases o H 20 o o H+ + OH-
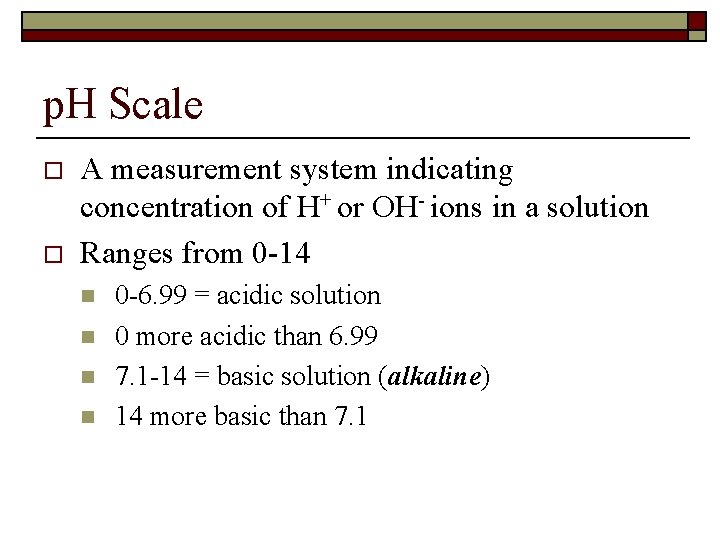
p. H Scale o o A measurement system indicating concentration of H+ or OH- ions in a solution Ranges from 0 -14 n n 0 -6. 99 = acidic solution 0 more acidic than 6. 99 7. 1 -14 = basic solution (alkaline) 14 more basic than 7. 1
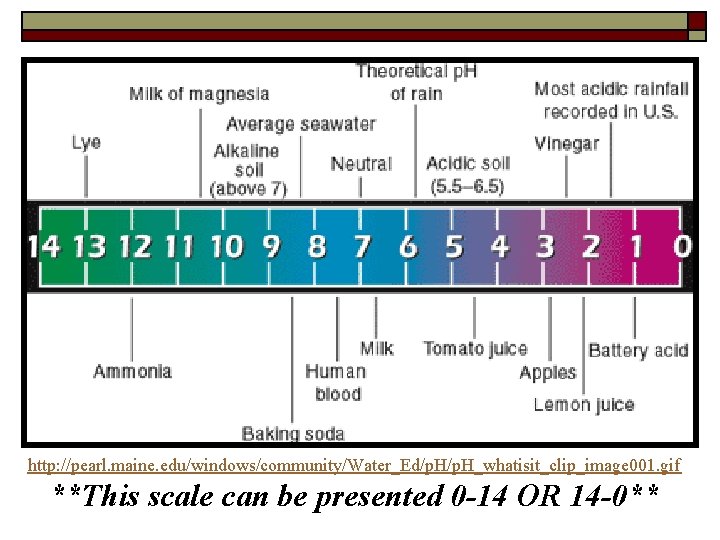
http: //pearl. maine. edu/windows/community/Water_Ed/p. H_whatisit_clip_image 001. gif **This scale can be presented 0 -14 OR 14 -0**
- Slides: 26