Biology Basic Chemistry Matter and Atomic Structure o
Biology Basic Chemistry
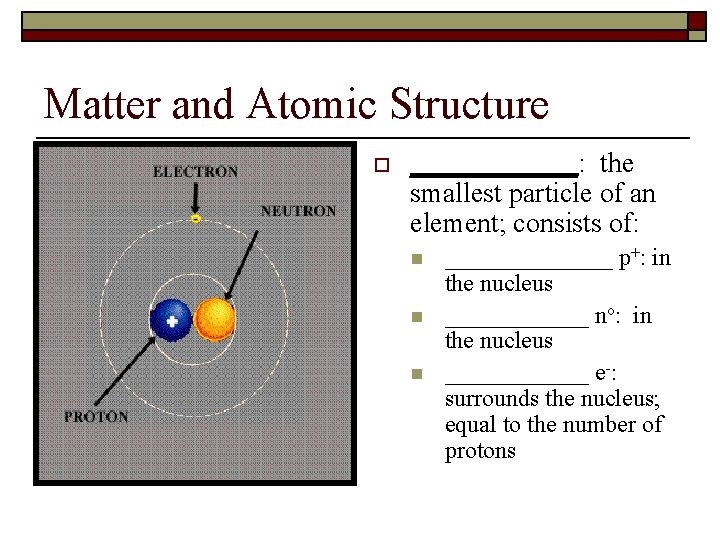
Matter and Atomic Structure o ______: the smallest particle of an element; consists of: n n n _______ p+: in the nucleus ______ no: in the nucleus ______ e-: surrounds the nucleus; equal to the number of protons
Matter and Atomic Structure o o _______: anything that has volume and mass _______: a substance not broken down into simpler substances by physical or chemical means n Each element has a 1 or 2 -letter symbol o o _______: A molecule is formed when two or more atoms join together chemically. n o Examples: oxygen (O), sodium (Na) Ex: hydrogen (H 2), oxygen (O 2) and nitrogen (N 2) _______: a molecule composed of atoms of 2+ different elements that are chemically combined n Ex: Na. Cl: salt, H 2 O: water
Matter and Atomic Structure o o __________: the number of protons in an atom’s nucleus __________: the number of protons and neutrons in an atom __________: the area of an atom surrounding the nucleus where electrons are found # of protons always equals the # of electrons; atoms have NO CHARGE
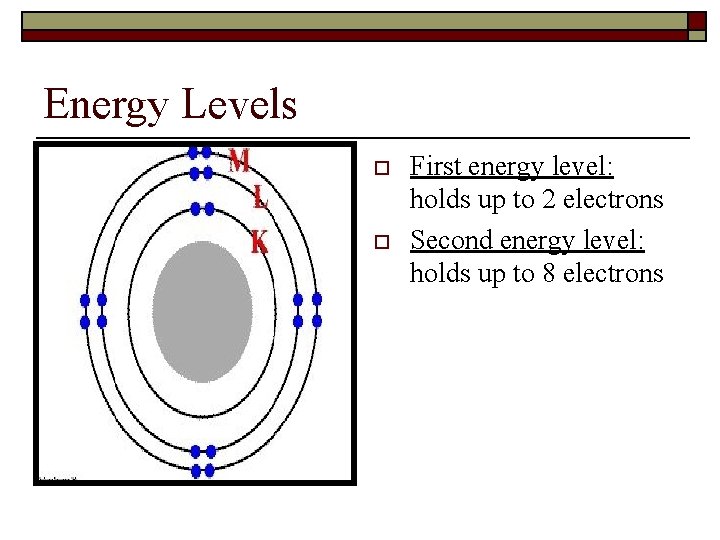
Energy Levels o o First energy level: holds up to 2 electrons Second energy level: holds up to 8 electrons
Chemical Bonds The main types of chemical bonds are: o ____________________ Copyright Pearson Prentice Hall
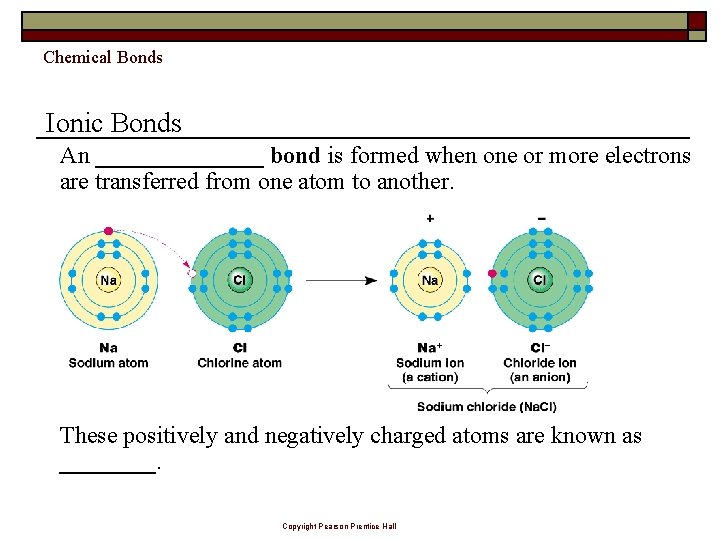
Chemical Bonds Ionic Bonds An _______ bond is formed when one or more electrons are transferred from one atom to another. These positively and negatively charged atoms are known as ____. Copyright Pearson Prentice Hall
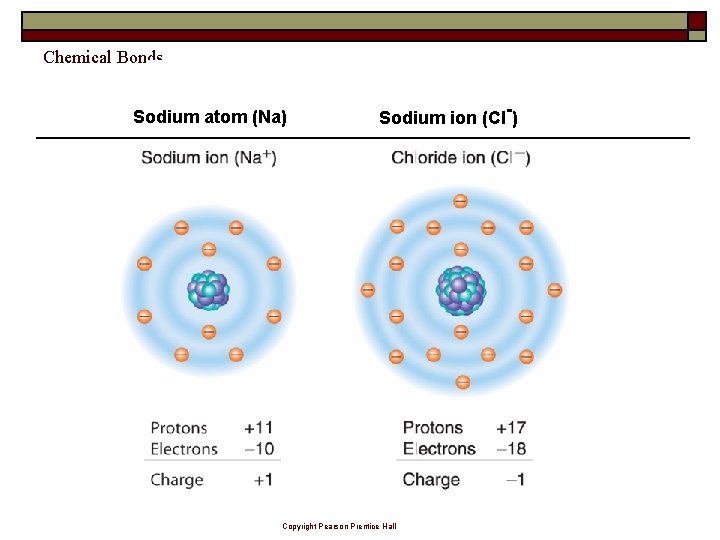
Chemical Bonds Sodium atom (Na) Sodium ion (Cl-) Copyright Pearson Prentice Hall
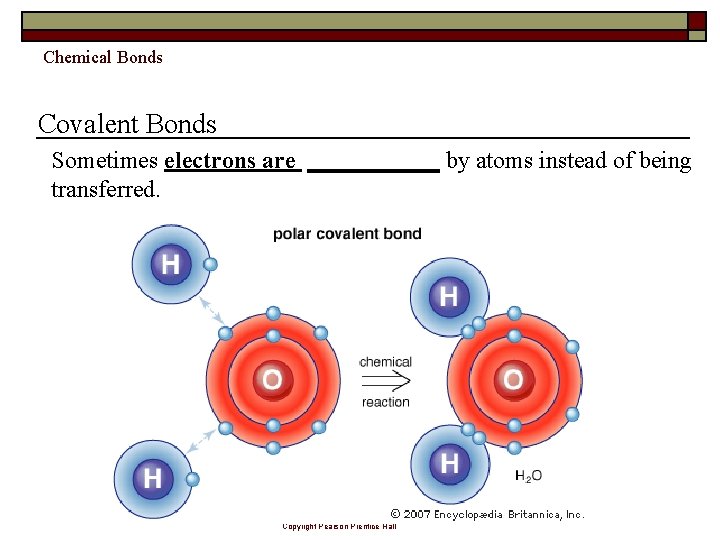
Chemical Bonds Covalent Bonds Sometimes electrons are ______ by atoms instead of being transferred. Copyright Pearson Prentice Hall
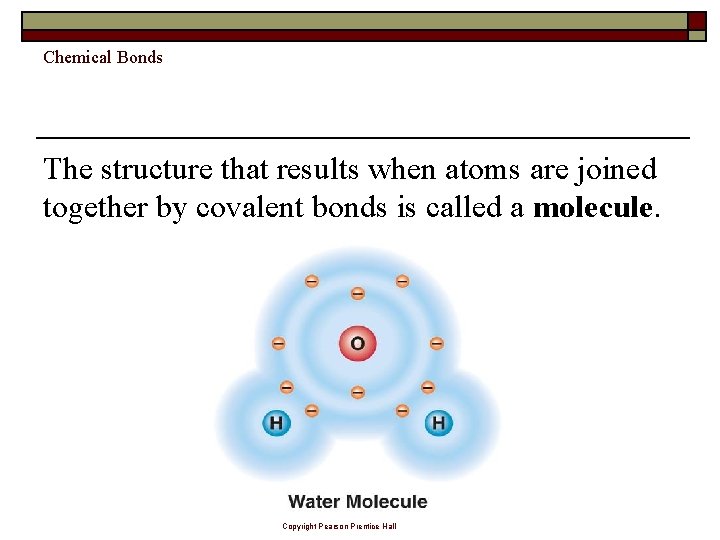
Chemical Bonds The structure that results when atoms are joined together by covalent bonds is called a molecule. Copyright Pearson Prentice Hall

Water o About 60 -90 percent of an organism is water Water is used in most reactions in the body Water is called the _____ solvent
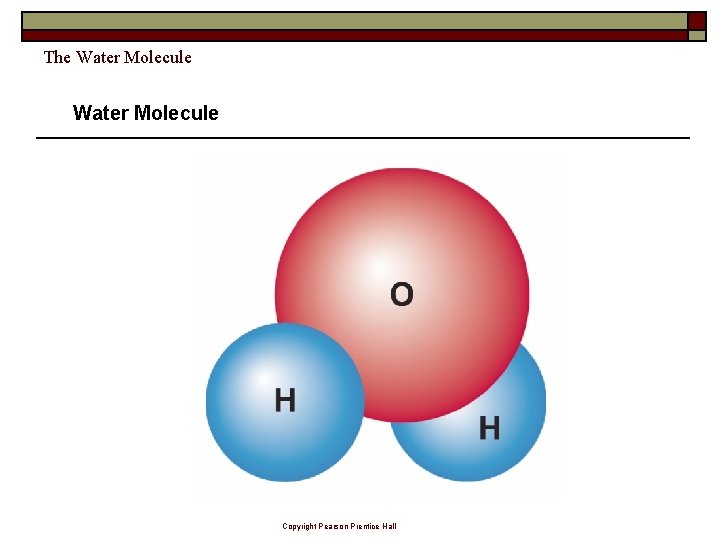
The Water Molecule Copyright Pearson Prentice Hall
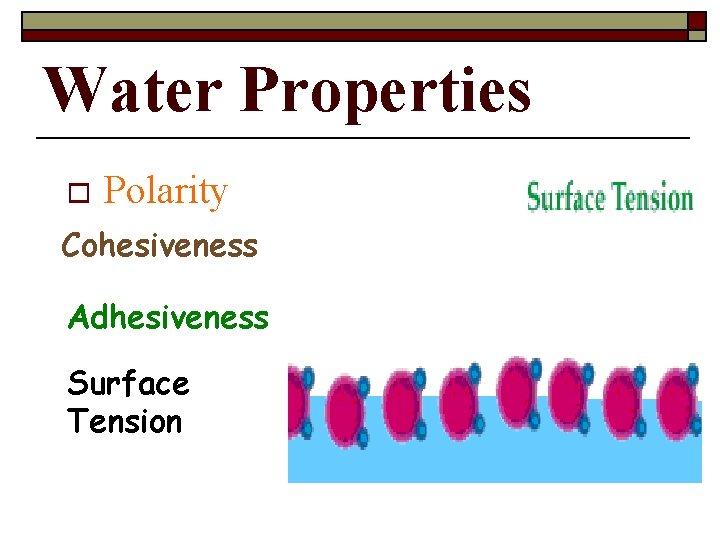
Water Properties o Polarity Cohesiveness Adhesiveness Surface Tension
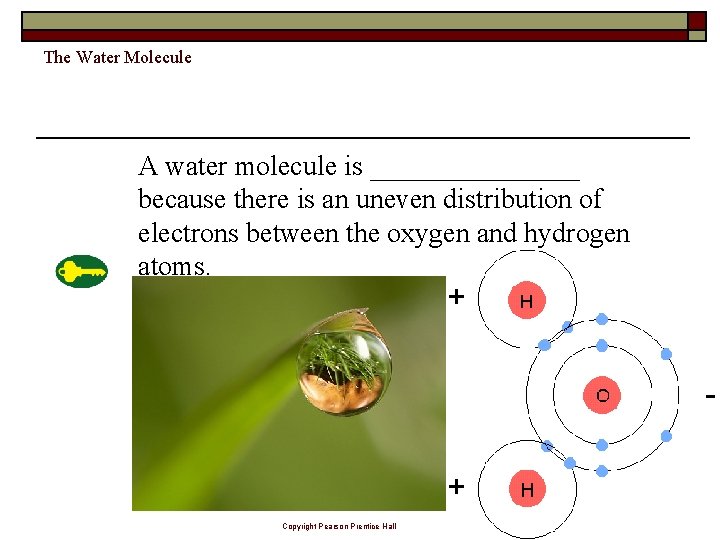
The Water Molecule A water molecule is ________ because there is an uneven distribution of electrons between the oxygen and hydrogen atoms. Copyright Pearson Prentice Hall

The Water Molecule _________ is an attraction between molecules of the same substance. Because of hydrogen bonding, water is extremely cohesive. n. Example: surface tension (bugs walking on water) Copyright Pearson Prentice Hall

The Water Molecule _________ is an attraction between molecules of different substances. Copyright Pearson Prentice Hall

Solutions and Suspensions A ________ is a material composed of two or more elements or compounds that are physically mixed but not chemically combined. Copyright Pearson Prentice Hall
Solutions and Suspensions Two types of mixtures can be made with water n __________ Copyright Pearson Prentice Hall

Solutions and Suspensions Solutions All the components of a ______ are evenly distributed throughout the solution. _______—the substance that is dissolved. _______—the substance in which the solute dissolves. Copyright Pearson Prentice Hall
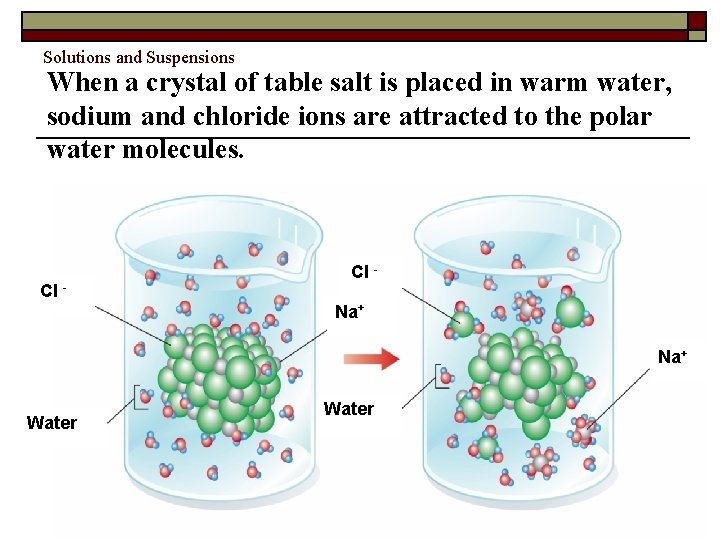
Solutions and Suspensions When a crystal of table salt is placed in warm water, sodium and chloride ions are attracted to the polar water molecules. Cl - Cl Na+ Water Copyright Pearson Prentice Hall
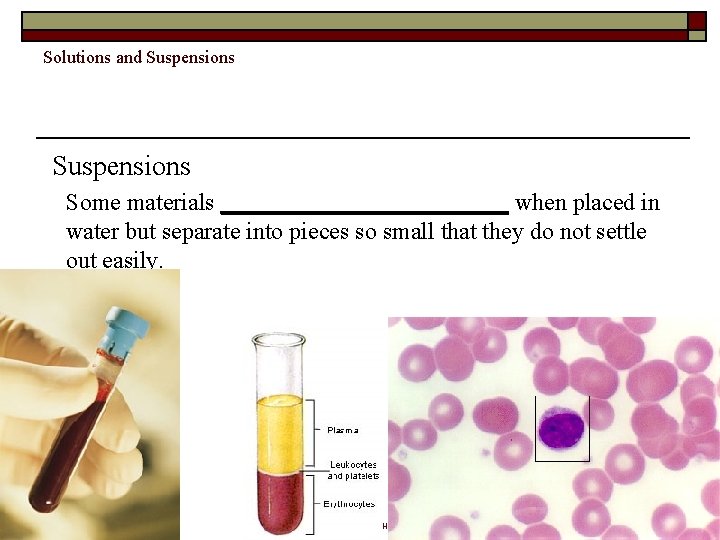
Solutions and Suspensions Some materials ____________ when placed in water but separate into pieces so small that they do not settle out easily. Copyright Pearson Prentice Hall
Acids & Bases o ______: any compound that forms H+ ions in solution ______: any compound that forms OH- in solution Water can dissociate to form acids and bases o H 20 o o H+ + OH-
p. H Scale o o A measurement system indicating concentration of H+ or OH- ions in a solution Ranges from ____ - _____ n n 0 -6. 99 = acidic solution 0 more acidic than 6. 99 7. 1 -14 = basic solution (alkaline) 14 more basic than 7. 1
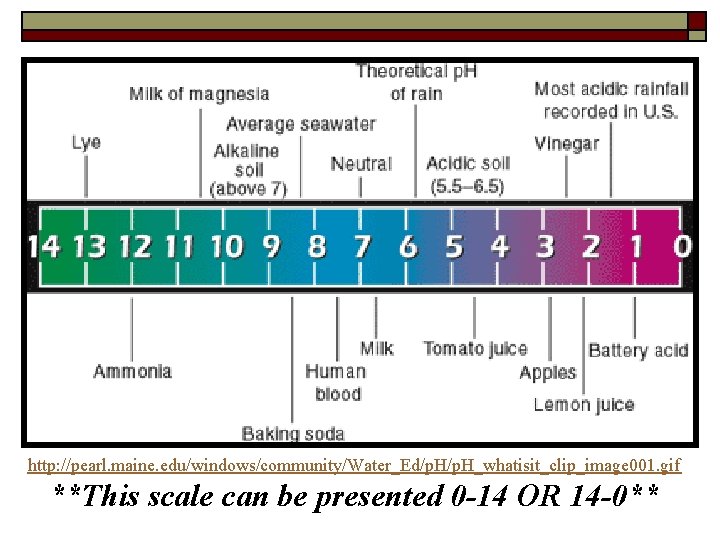
http: //pearl. maine. edu/windows/community/Water_Ed/p. H_whatisit_clip_image 001. gif **This scale can be presented 0 -14 OR 14 -0**
- Slides: 24