Biology 1111 K Lecture 2 Slide 2 particles

Biology 1111 K Lecture 2
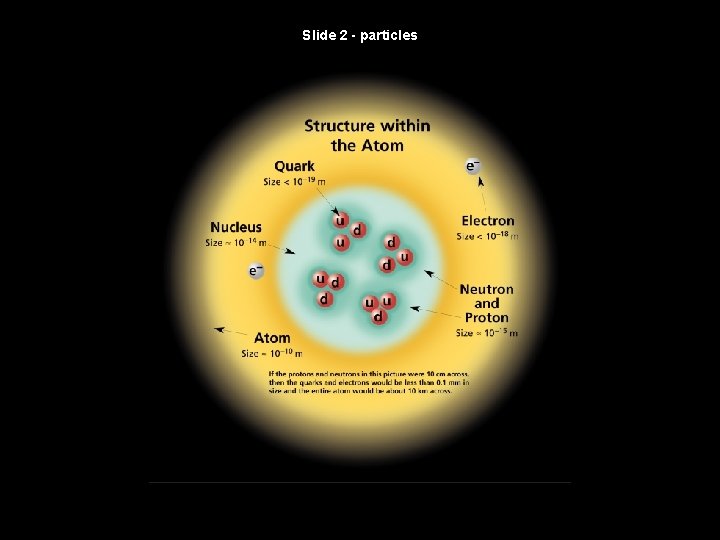
Slide 2 - particles

Slide 3 – particle charge
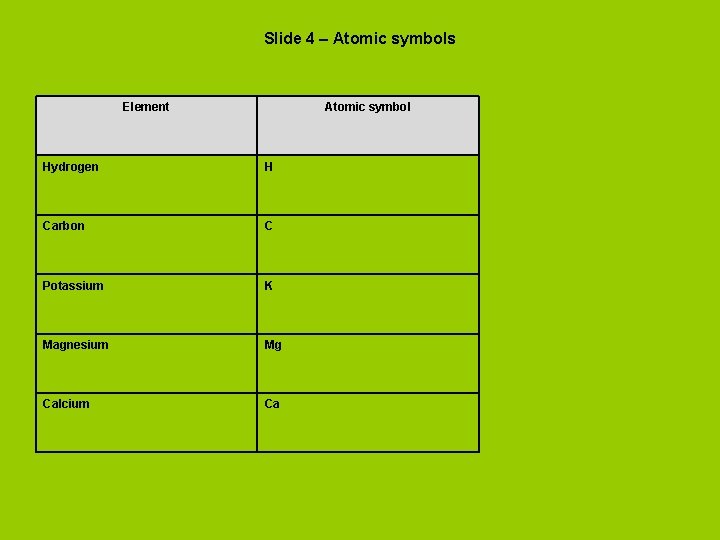
Slide 4 – Atomic symbols Element Atomic symbol Hydrogen H Carbon C Potassium K Magnesium Mg Calcium Ca
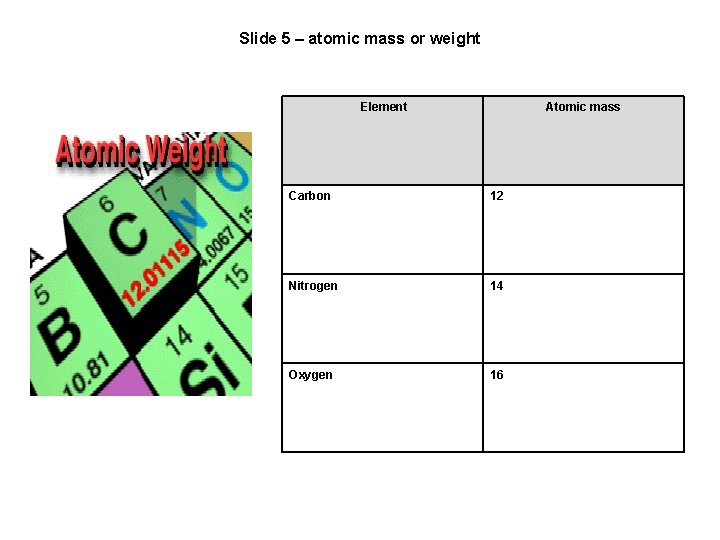
Slide 5 – atomic mass or weight Element Atomic mass Carbon 12 Nitrogen 14 Oxygen 16

Slide 6 – atomic number
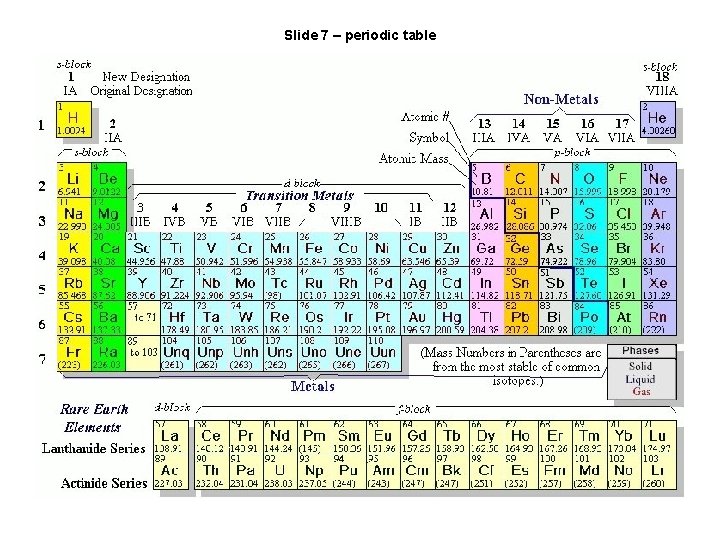
Slide 7 – periodic table
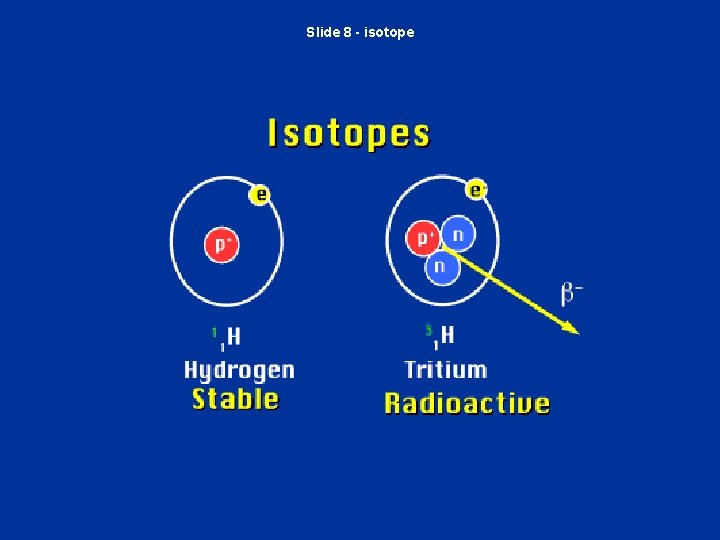
Slide 8 - isotope
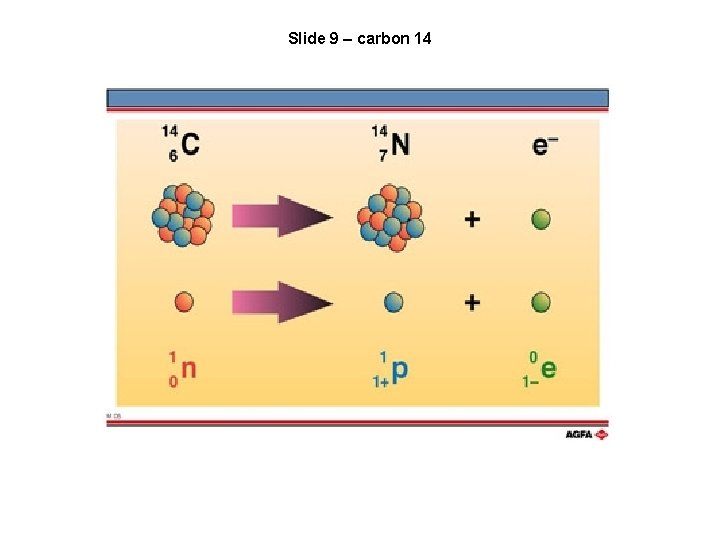
Slide 9 – carbon 14
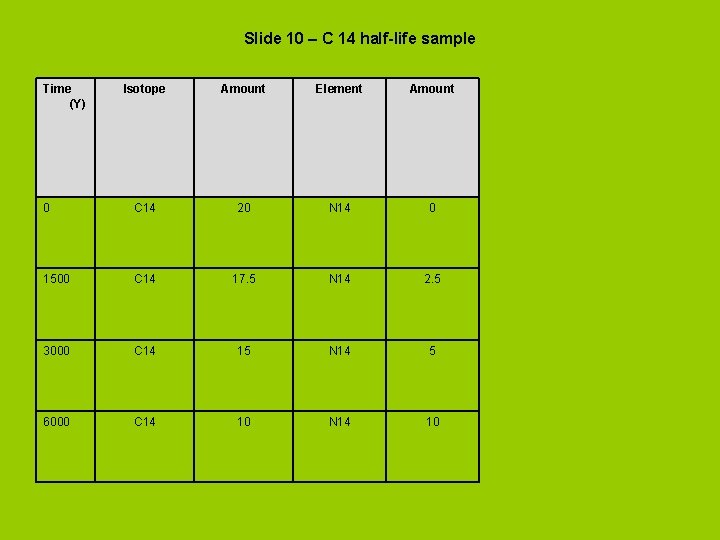
Slide 10 – C 14 half-life sample Time (Y) Isotope Amount Element Amount 0 C 14 20 N 14 0 1500 C 14 17. 5 N 14 2. 5 3000 C 14 15 N 14 5 6000 C 14 10 N 14 10
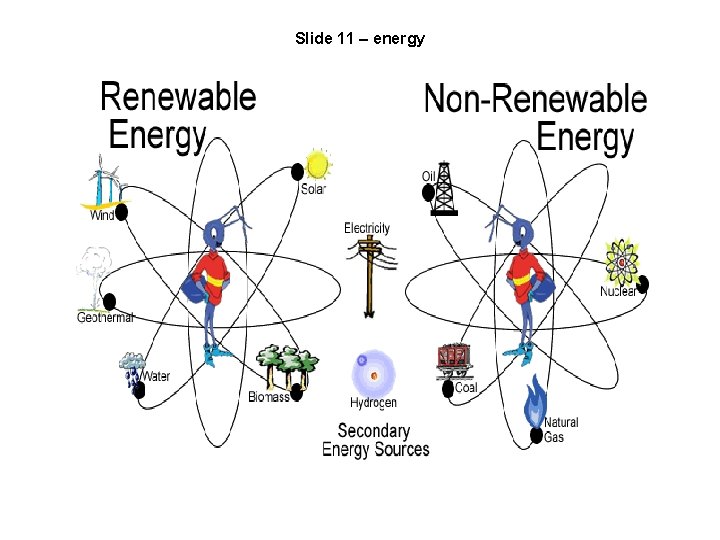
Slide 11 – energy
Slide 12 – stored energy
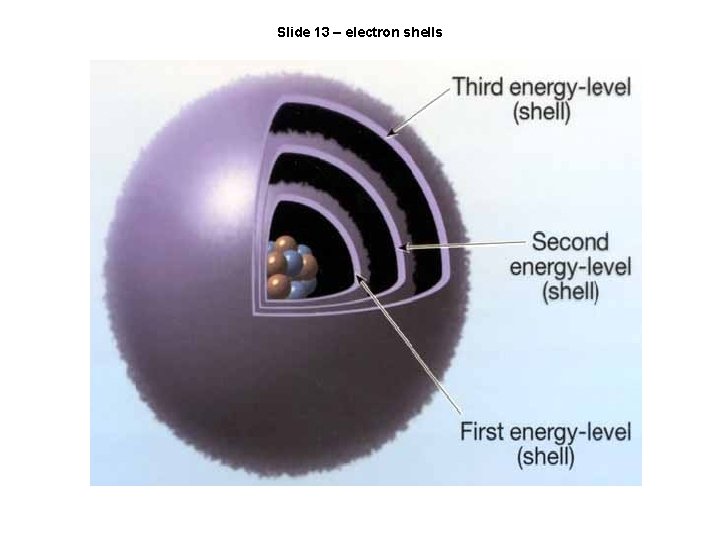
Slide 13 – electron shells
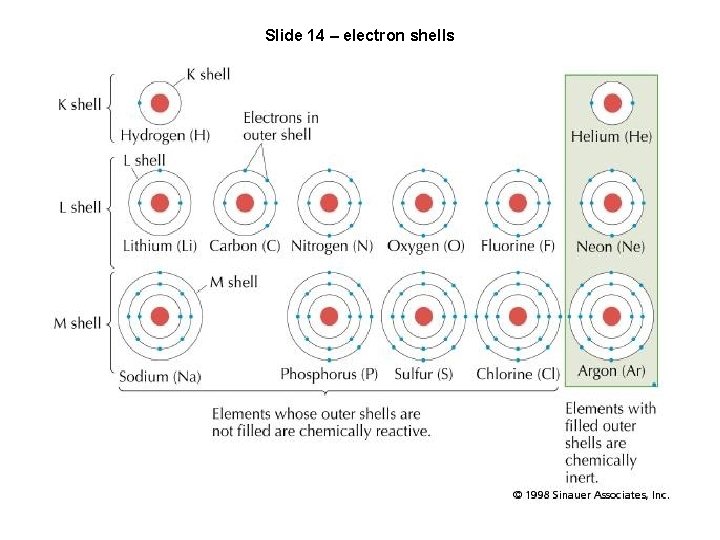
Slide 14 – electron shells
Slide 15 – octet rule

Slide 16 – chemical formulas H 2 O – water - 2 hydrogen and one oxygen CO 2 – carbon dioxide - 1 carbon, 2 oxygen H 2 SO 4 – sulfuric acid – 2 hydrogen, one sulfur, and four oxygen HCl – hydrochloric acid – one hydrogen, one chlorine
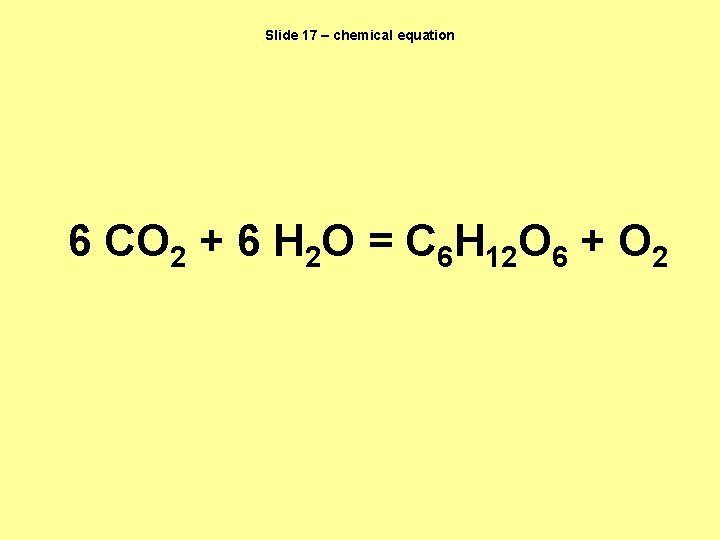
Slide 17 – chemical equation 6 CO 2 + 6 H 2 O = C 6 H 12 O 6 + O 2
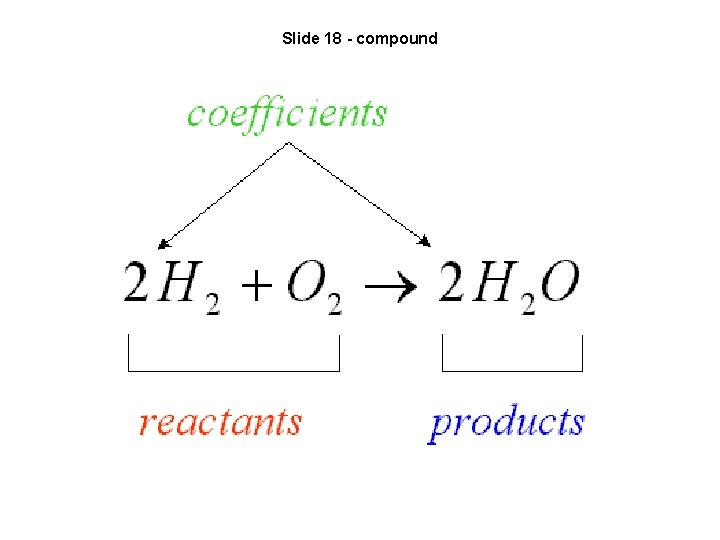
Slide 18 - compound
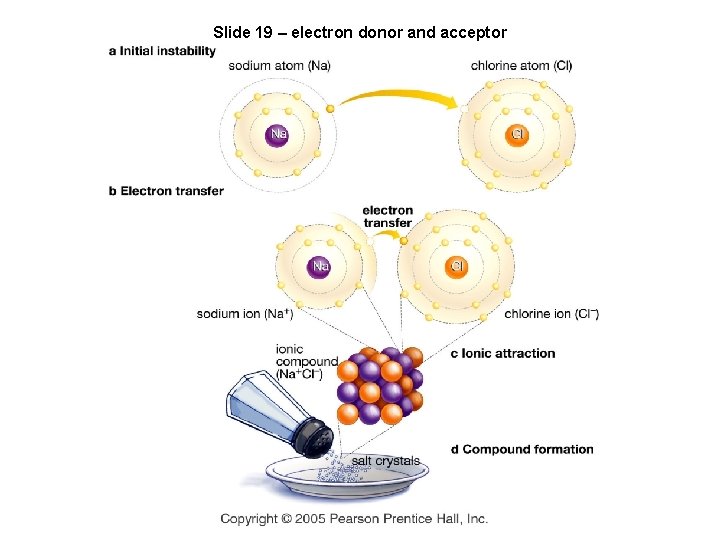
Slide 19 – electron donor and acceptor
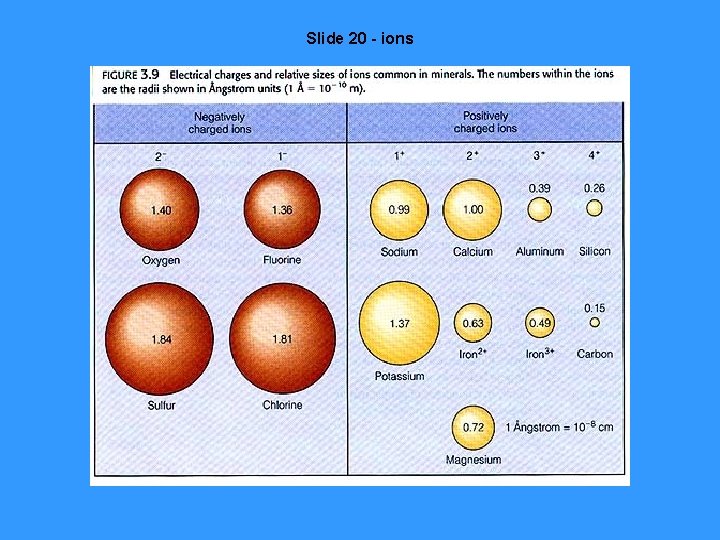
Slide 20 - ions
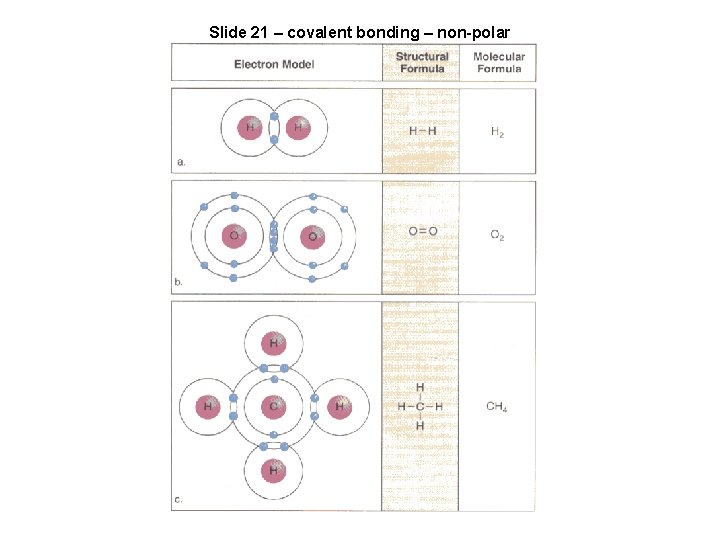
Slide 21 – covalent bonding – non-polar
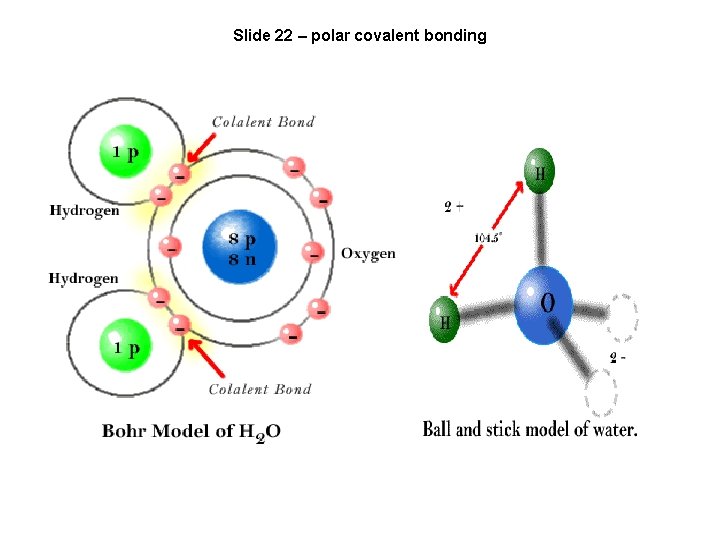
Slide 22 – polar covalent bonding
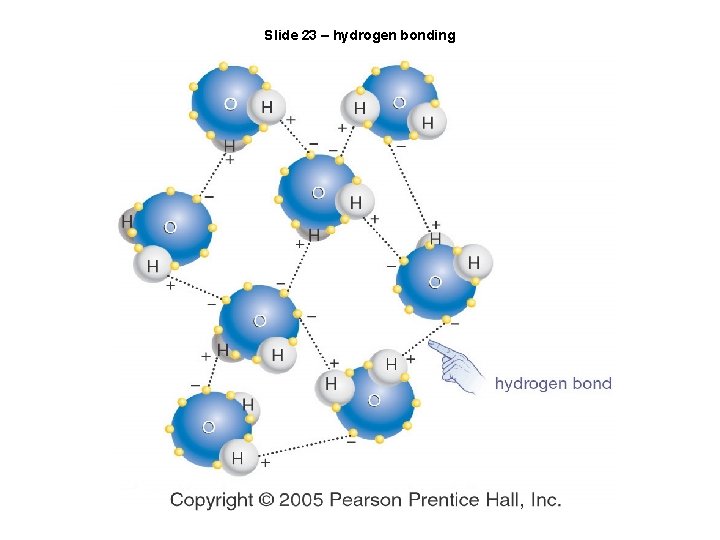
Slide 23 – hydrogen bonding
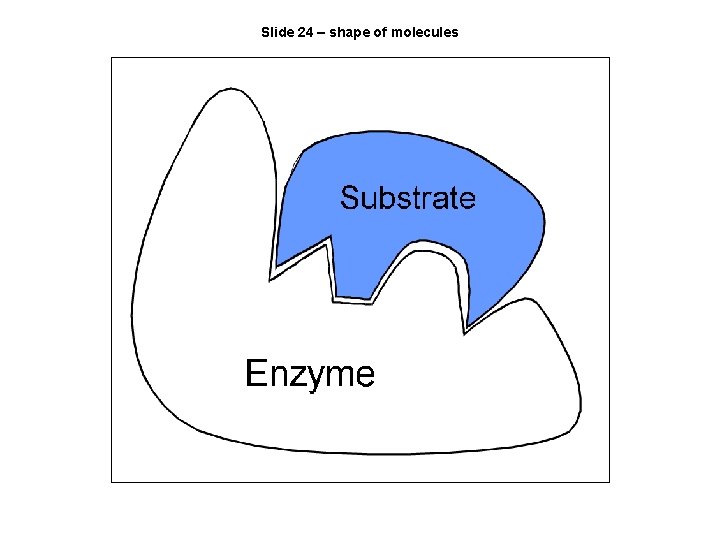
Slide 24 – shape of molecules

Slide 25 – acids and bases Acids – molecules that dissociates in water and releases hydrogen ions (H+). When dissociation is complete, the acid is called a strong acid. HCl H+ and OHH 2 SO 4 H+ and HSO 4 Bases – molecules that either takes up hydrogen ions (H+) or releases hydroxide ions (OH-). When dissociation is complete, the base is known as a strong base. Na. OH Na + and OH – Pure water is neutral since in its rare ionic form it gives off equal numbers of hydrogen and hydroxide ions. H 2 O H+ and OH-
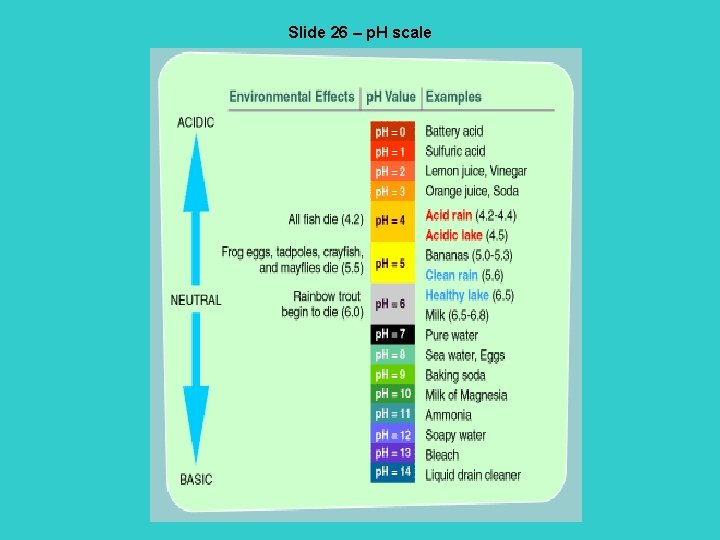
Slide 26 – p. H scale
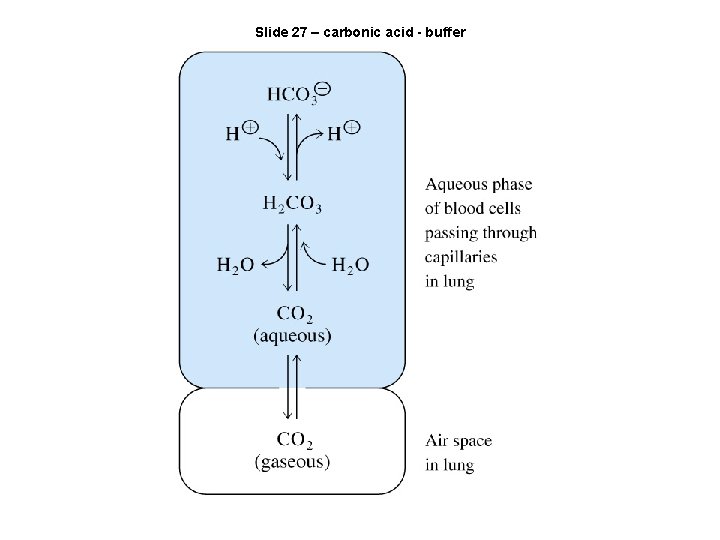
Slide 27 – carbonic acid - buffer
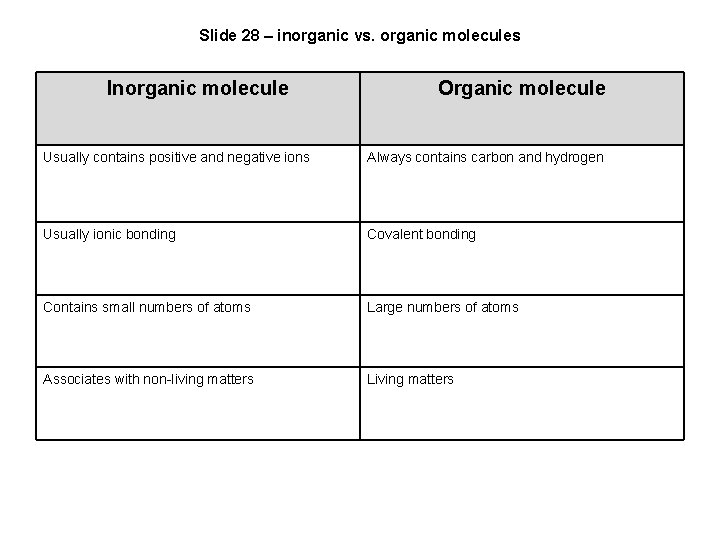
Slide 28 – inorganic vs. organic molecules Inorganic molecule Organic molecule Usually contains positive and negative ions Always contains carbon and hydrogen Usually ionic bonding Covalent bonding Contains small numbers of atoms Large numbers of atoms Associates with non-living matters Living matters
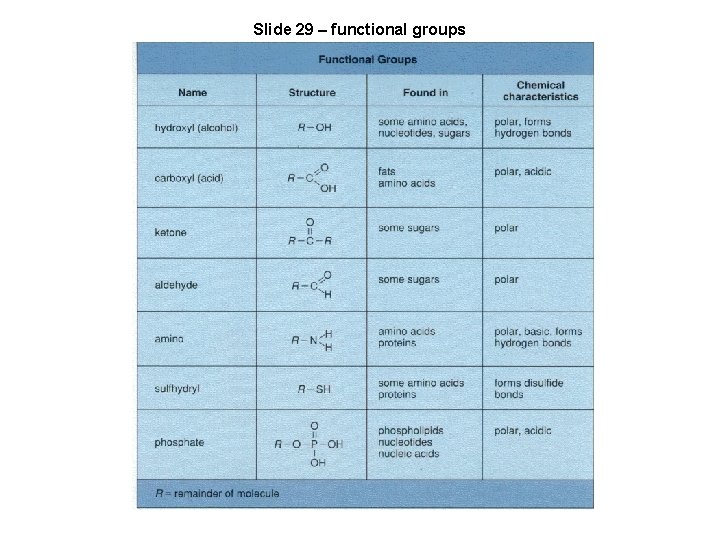
Slide 29 – functional groups
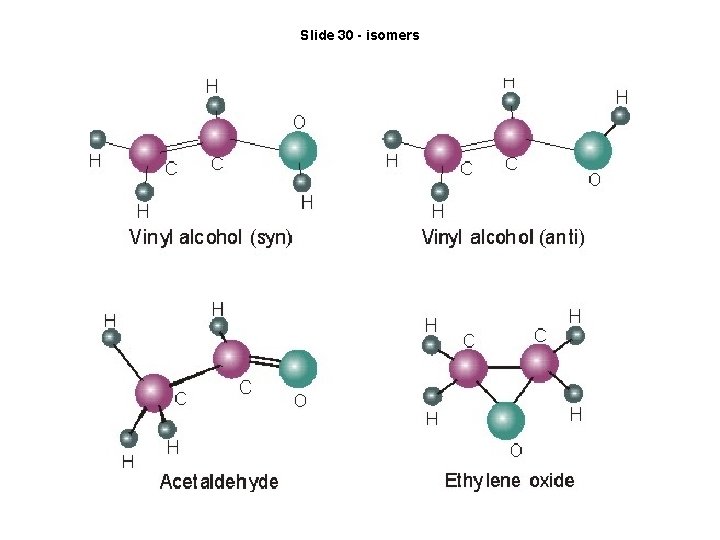
Slide 30 - isomers
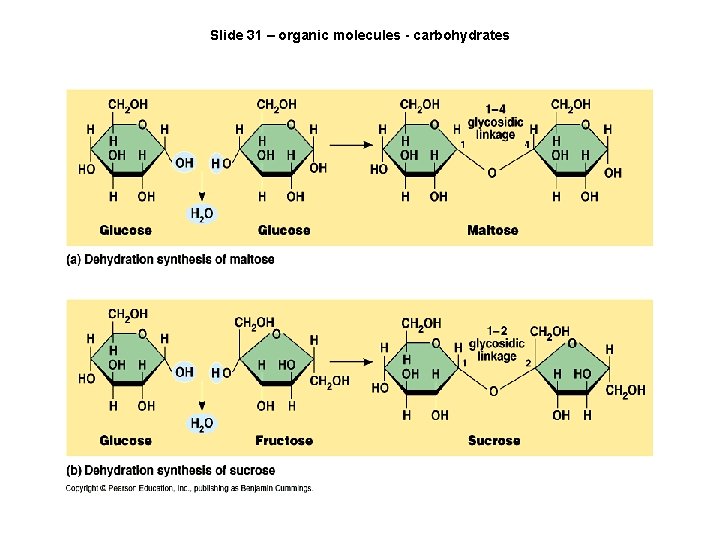
Slide 31 – organic molecules - carbohydrates
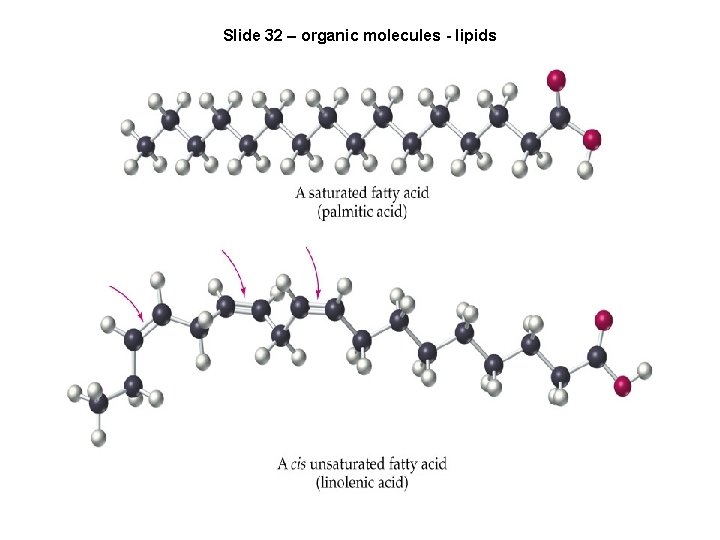
Slide 32 – organic molecules - lipids
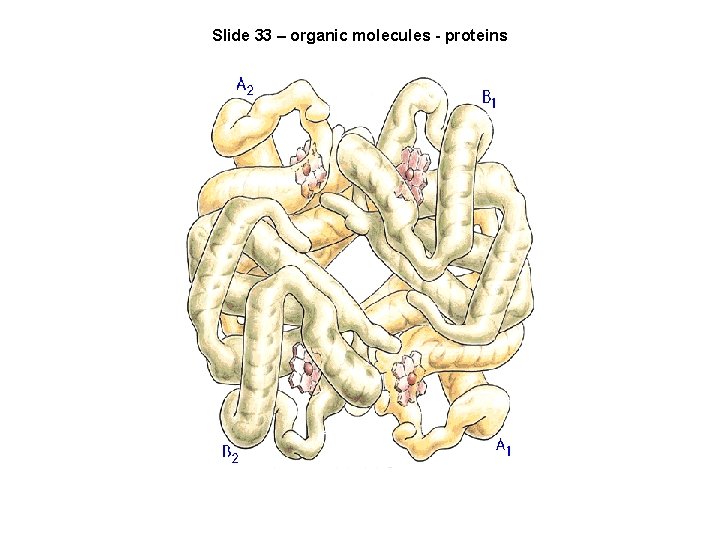
Slide 33 – organic molecules - proteins
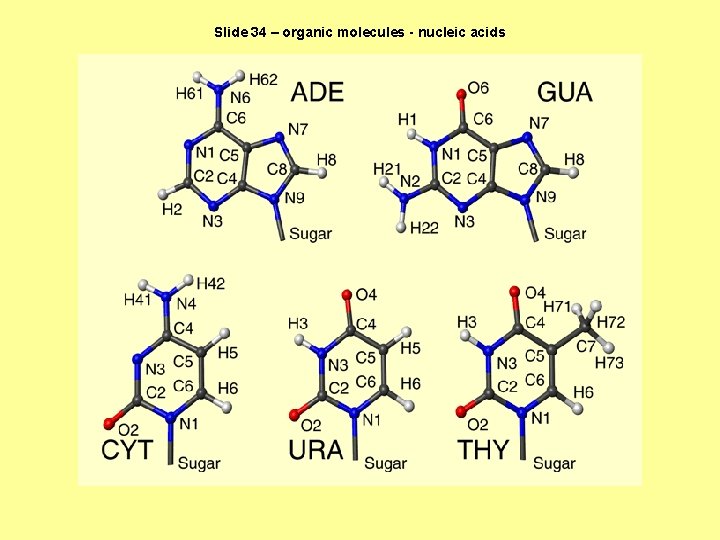
Slide 34 – organic molecules - nucleic acids
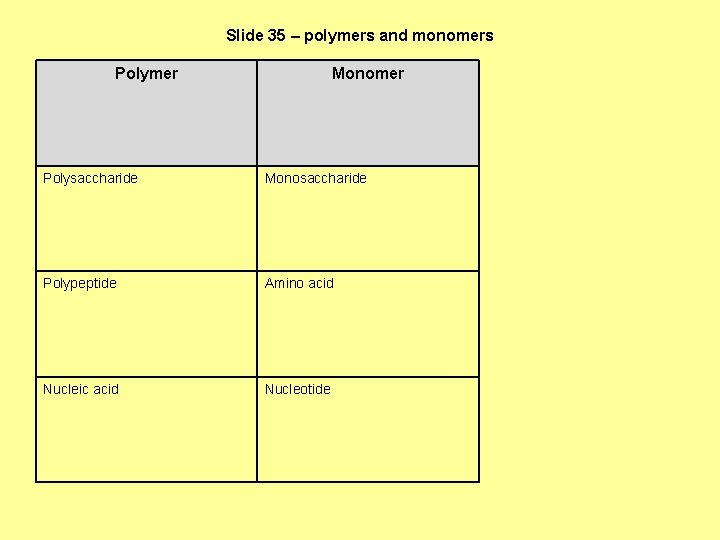
Slide 35 – polymers and monomers Polymer Monomer Polysaccharide Monosaccharide Polypeptide Amino acid Nucleic acid Nucleotide
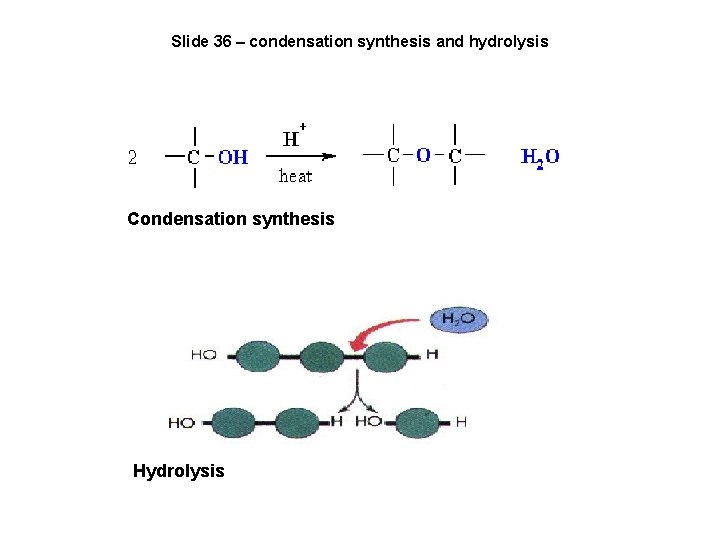
Slide 36 – condensation synthesis and hydrolysis Condensation synthesis Hydrolysis
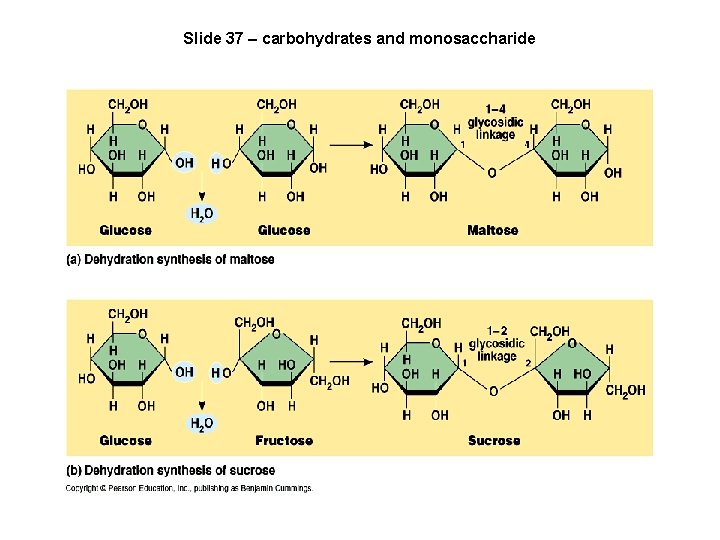
Slide 37 – carbohydrates and monosaccharide
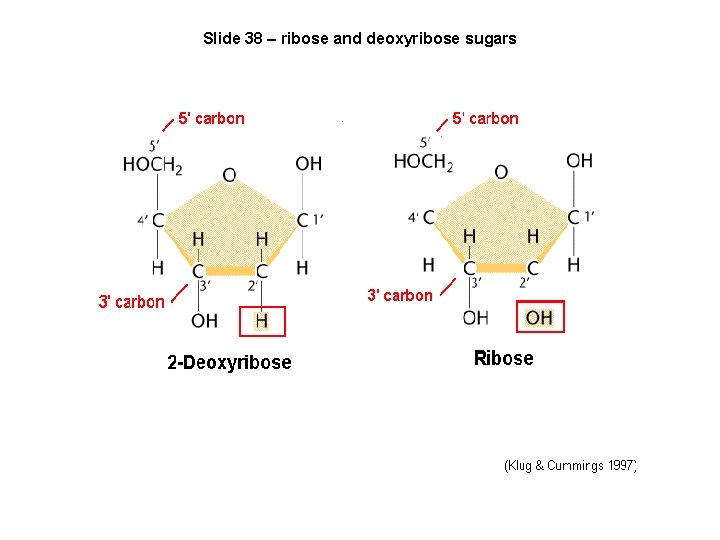
Slide 38 – ribose and deoxyribose sugars
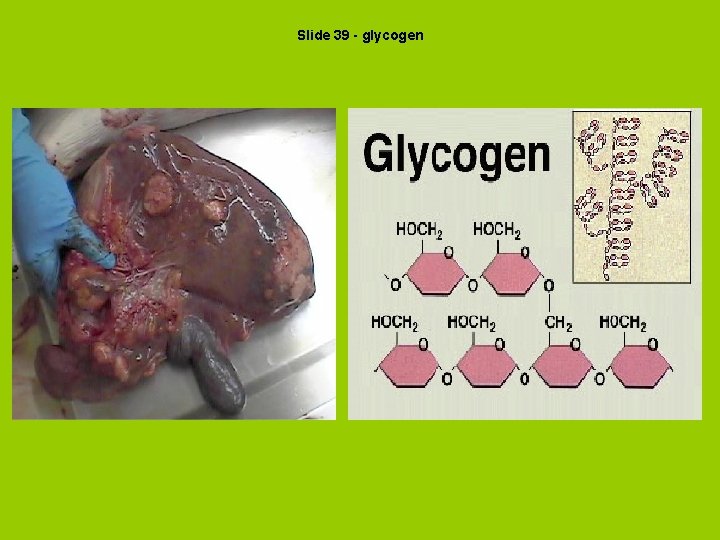
Slide 39 - glycogen
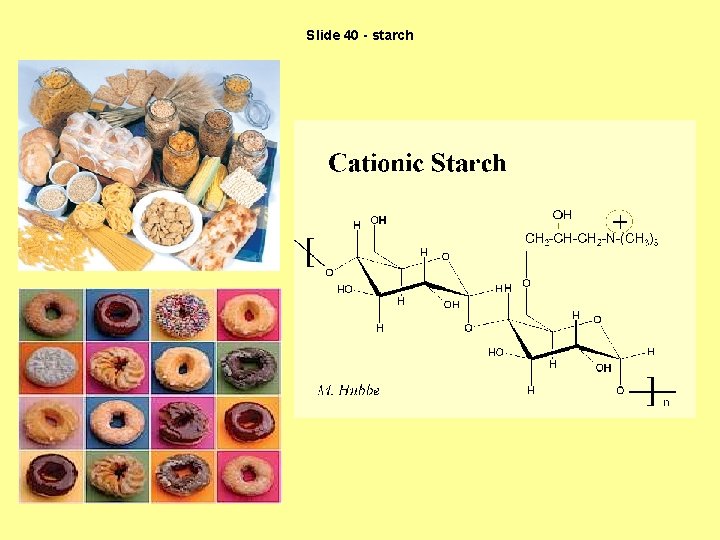
Slide 40 - starch
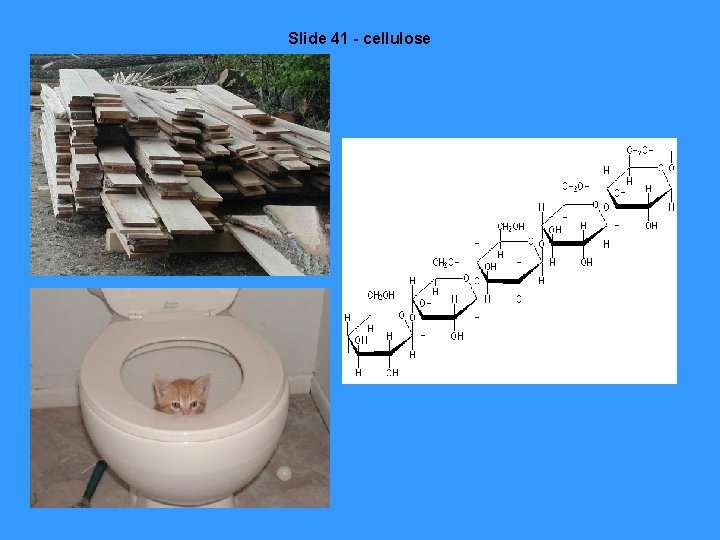
Slide 41 - cellulose
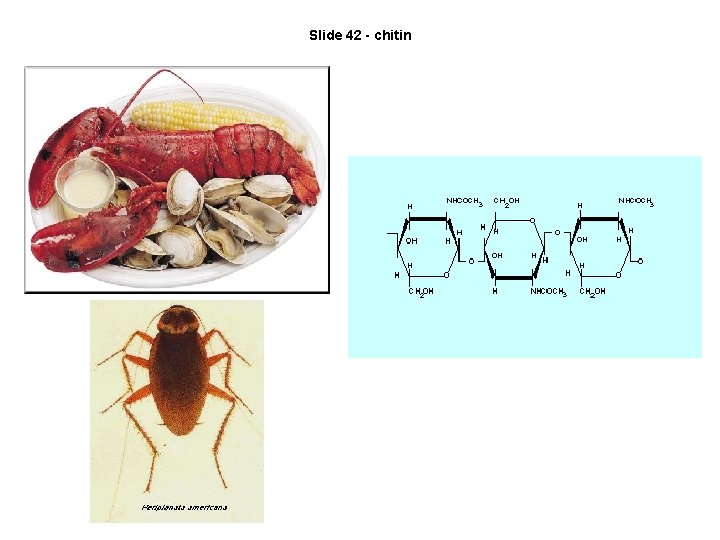
Slide 42 - chitin
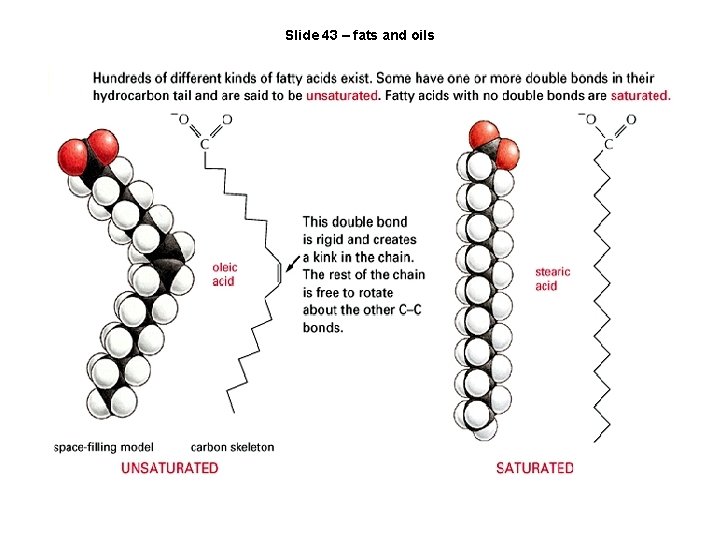
Slide 43 – fats and oils
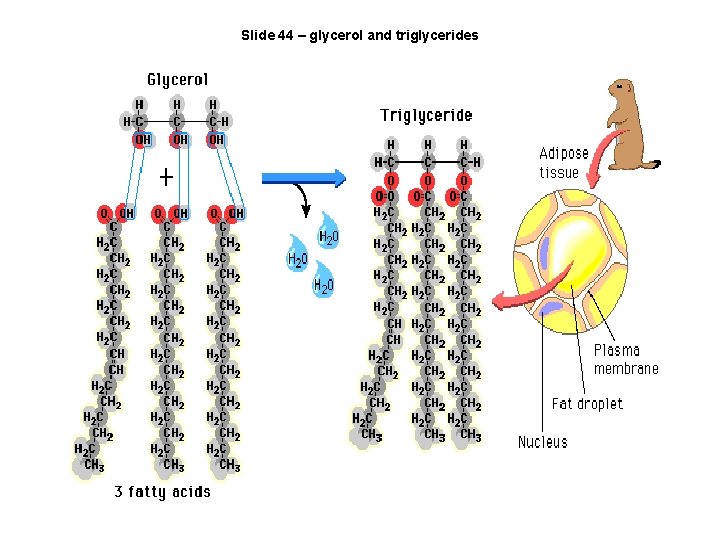
Slide 44 – glycerol and triglycerides
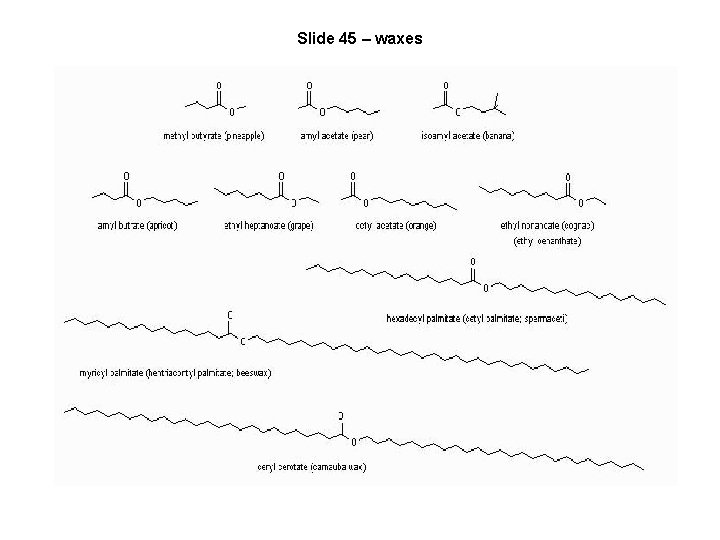
Slide 45 – waxes
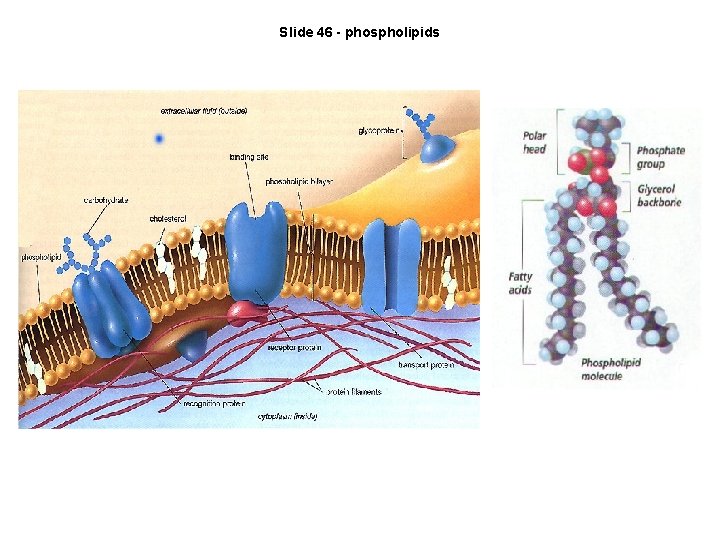
Slide 46 - phospholipids
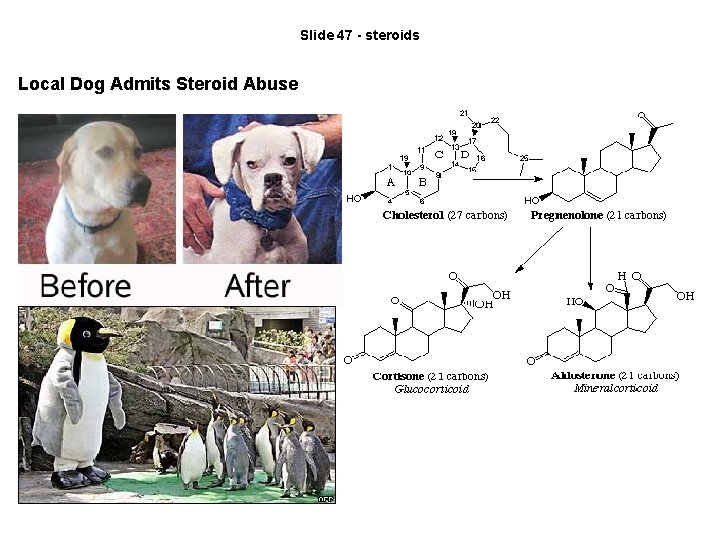
Slide 47 - steroids Local Dog Admits Steroid Abuse
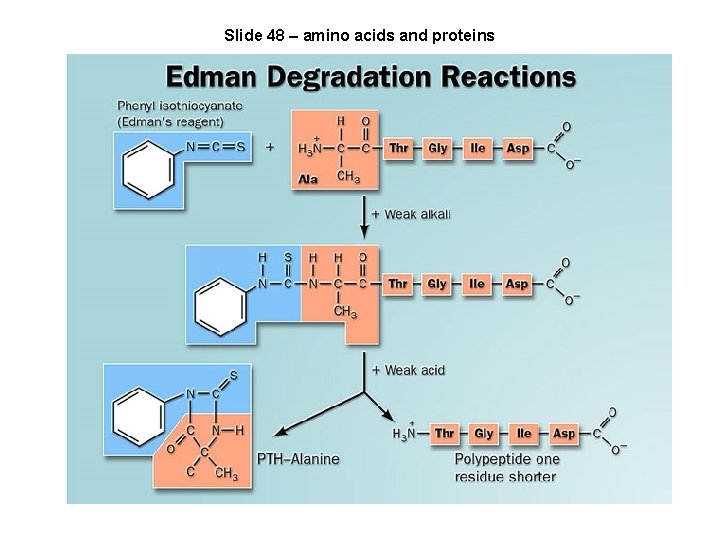
Slide 48 – amino acids and proteins
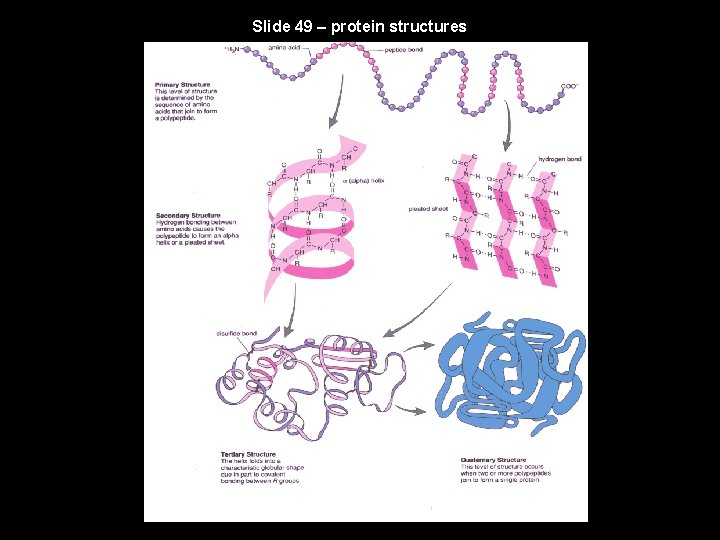
Slide 49 – protein structures
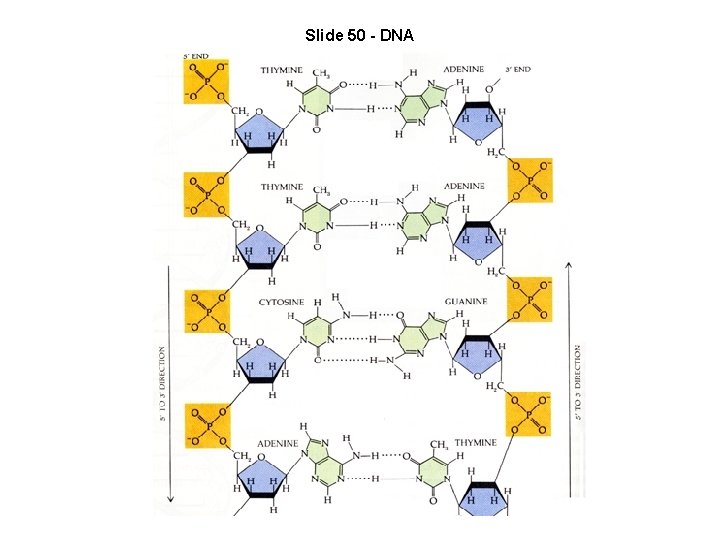
Slide 50 - DNA
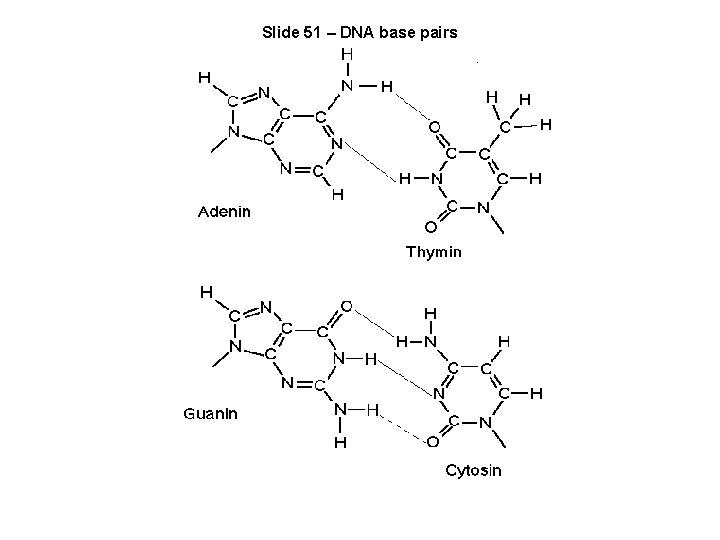
Slide 51 – DNA base pairs
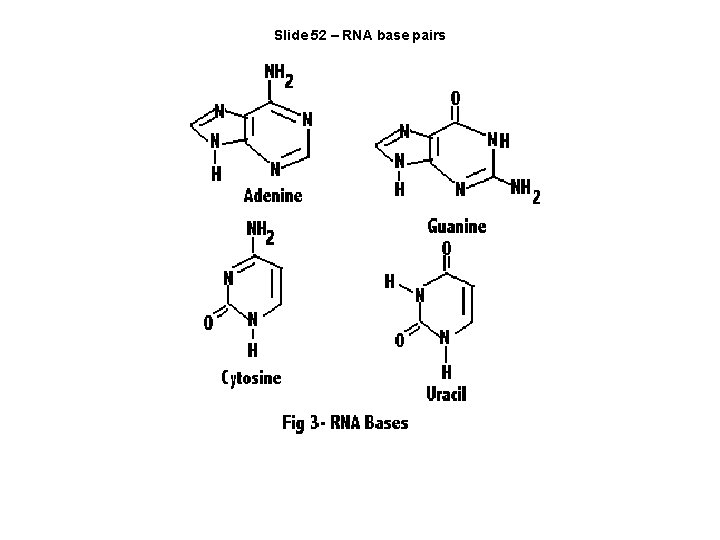
Slide 52 – RNA base pairs
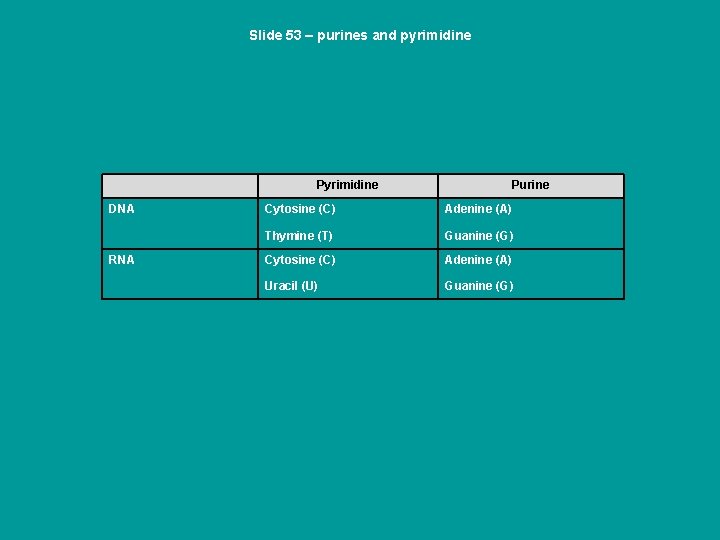
Slide 53 – purines and pyrimidine Pyrimidine DNA RNA Purine Cytosine (C) Adenine (A) Thymine (T) Guanine (G) Cytosine (C) Adenine (A) Uracil (U) Guanine (G)
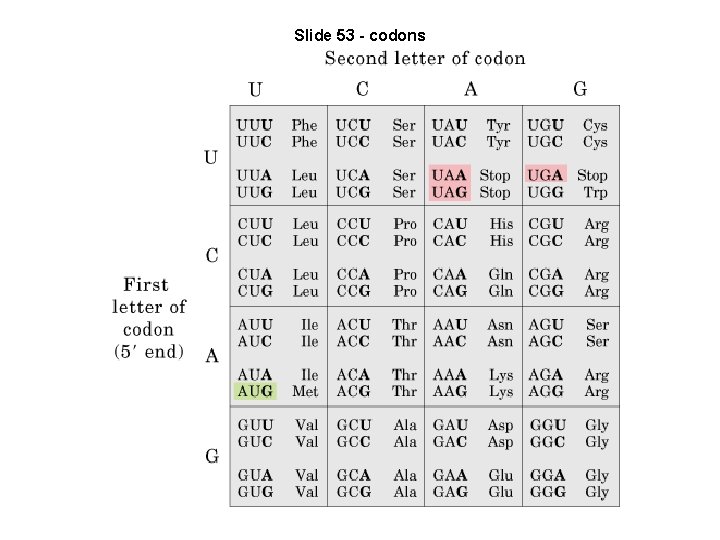
Slide 53 - codons
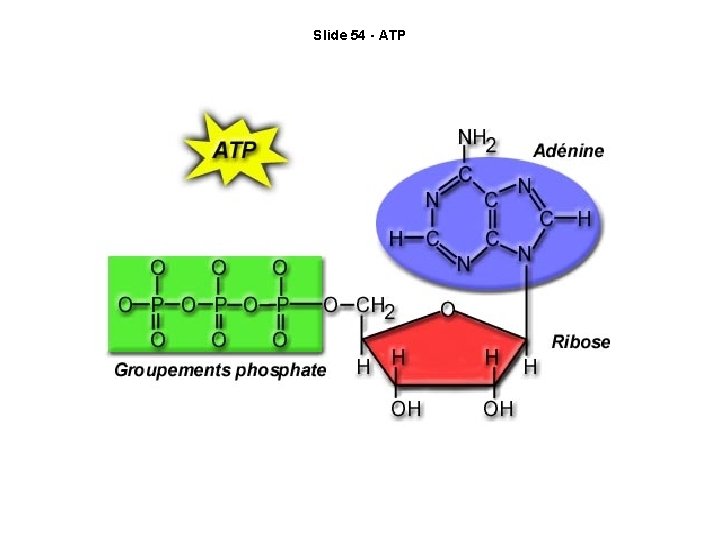
Slide 54 - ATP
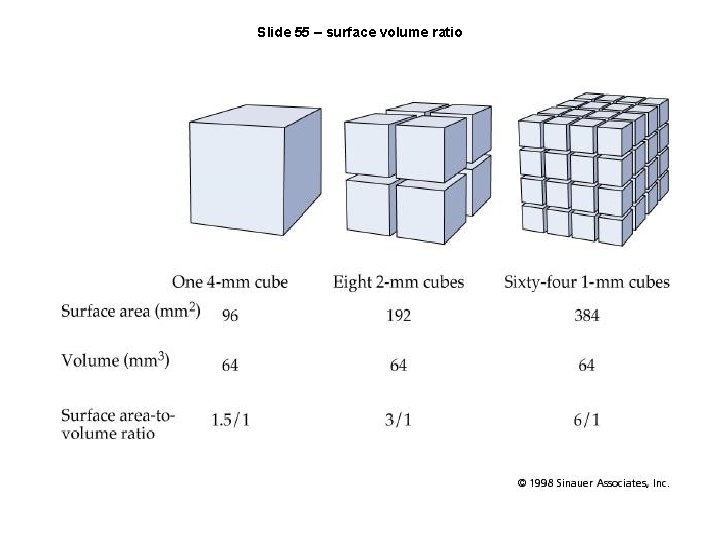
Slide 55 – surface volume ratio
- Slides: 56