Biologically Important Molecules Glycine AA I Water A
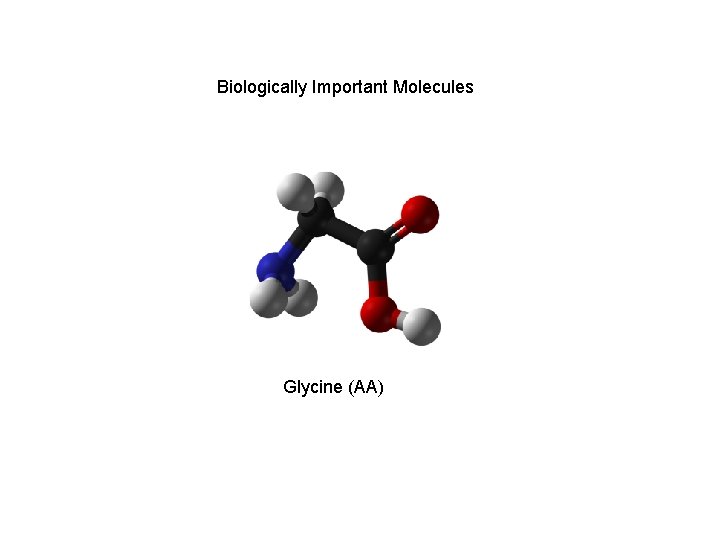
Biologically Important Molecules Glycine (AA)

I. Water A. Structure – did it B. Properties - Solvent High specific heat (thermally stable) High heat of vaporization Adhesive/cohesive

I. Water C. Water and Life on Earth is inconceivable without water. Life requires rapid and continuous chemical reactions facilitated by a dissolution of reactants in a liquid solvent. Water’s solvent properties are ideal. Water is a liquid over a wide temperature range that is very common on Earth. (High specific heat, vaporization). Water is abundant on Earth, covering over 70% of the surface. Water is a thermally stable internal/external environment. No surprize that life probably originated in water (3. 8 bya), and did not adapt to exploit the desiccating terrestrial environments until the last ~10% of Life’s history (0. 4 bya).

Biologically Important Molecules I. Water II. Carbohydrates
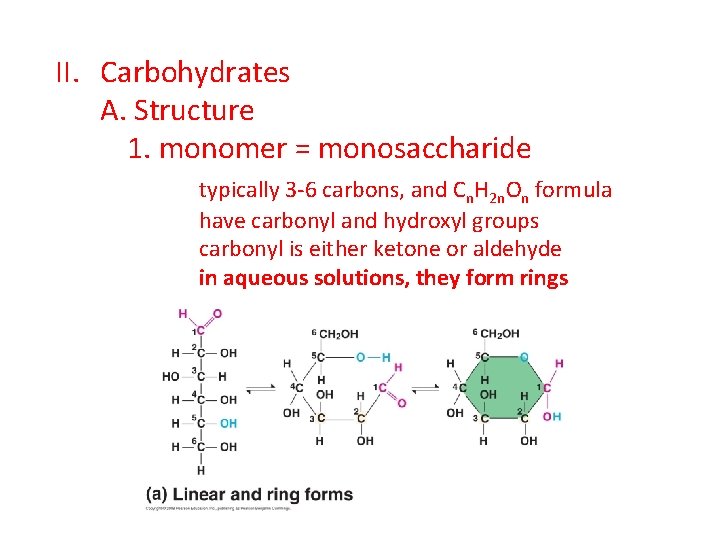
II. Carbohydrates A. Structure 1. monomer = monosaccharide typically 3 -6 carbons, and Cn. H 2 n. On formula have carbonyl and hydroxyl groups carbonyl is either ketone or aldehyde in aqueous solutions, they form rings
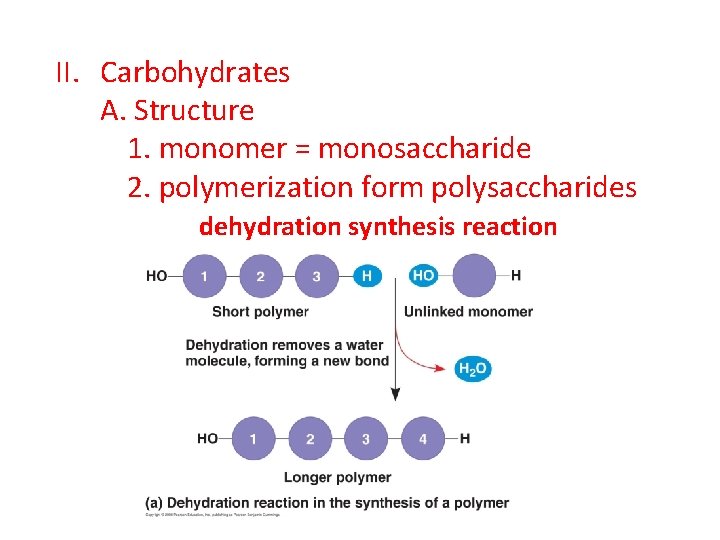
II. Carbohydrates A. Structure 1. monomer = monosaccharide 2. polymerization form polysaccharides dehydration synthesis reaction
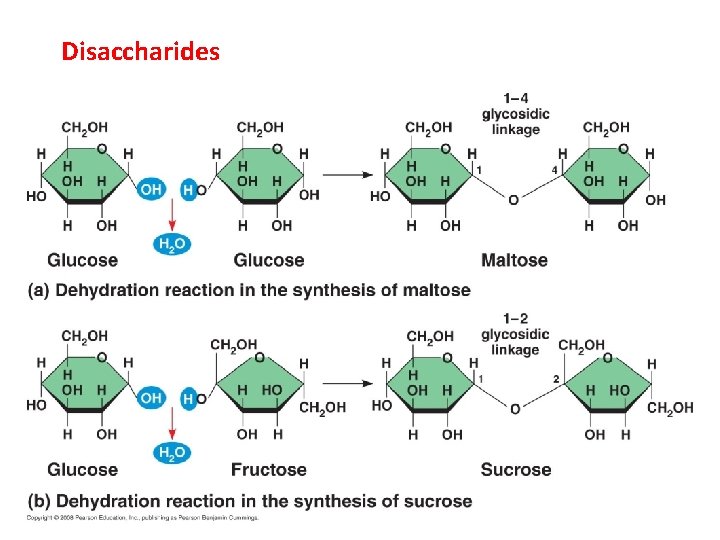
Disaccharides
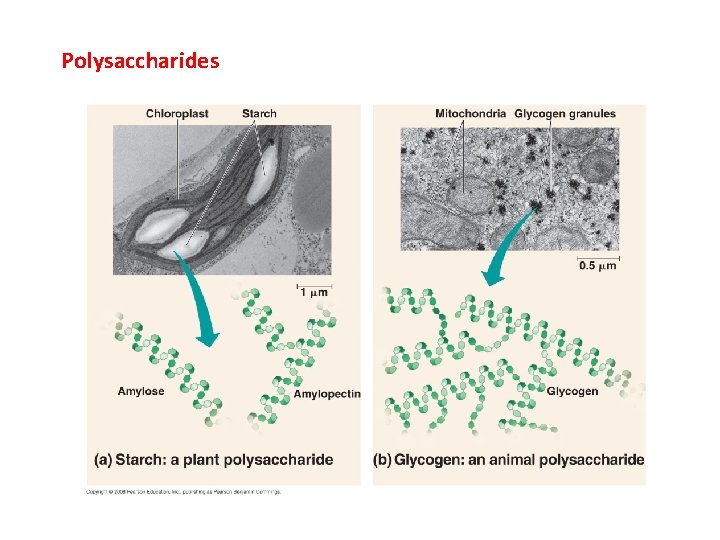
Polysaccharides
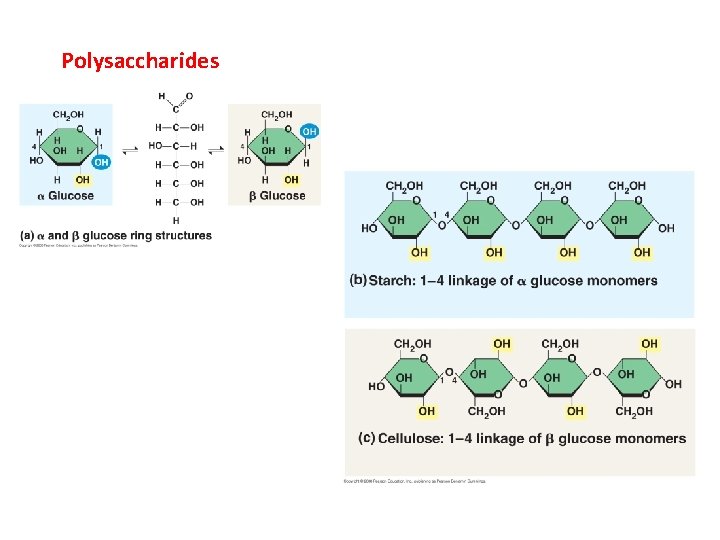
Polysaccharides
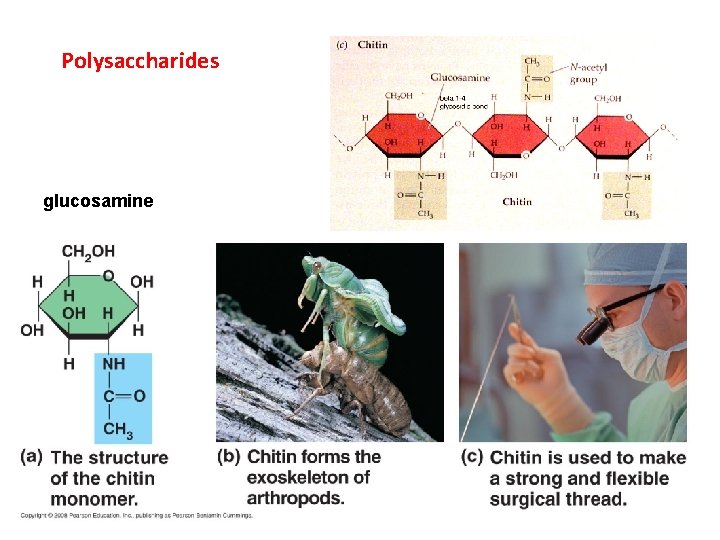
Polysaccharides glucosamine
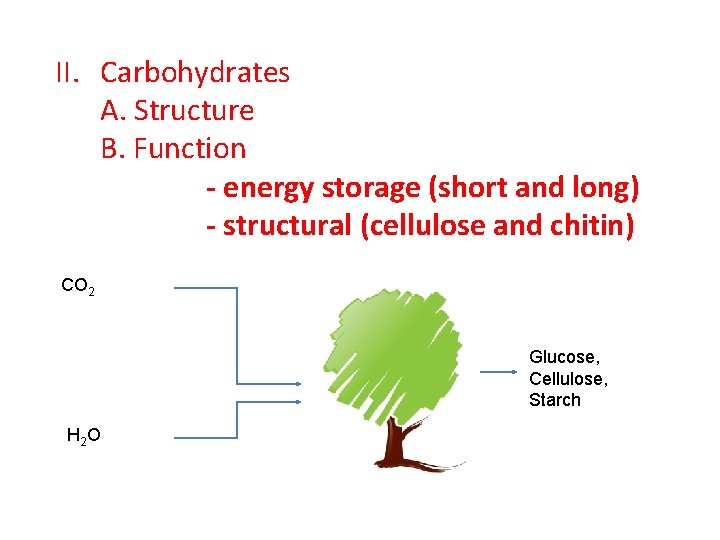
II. Carbohydrates A. Structure B. Function - energy storage (short and long) - structural (cellulose and chitin) CO 2 Glucose, Cellulose, Starch H 2 O
Biologically Important Molecules I. Water II. Carbohydrates III. Lipids

III. Lipids - not true polymers; an assortment of hydrophobic, hydrocarbon molecules classes as fats, phospholipids, waxes, or steroids.
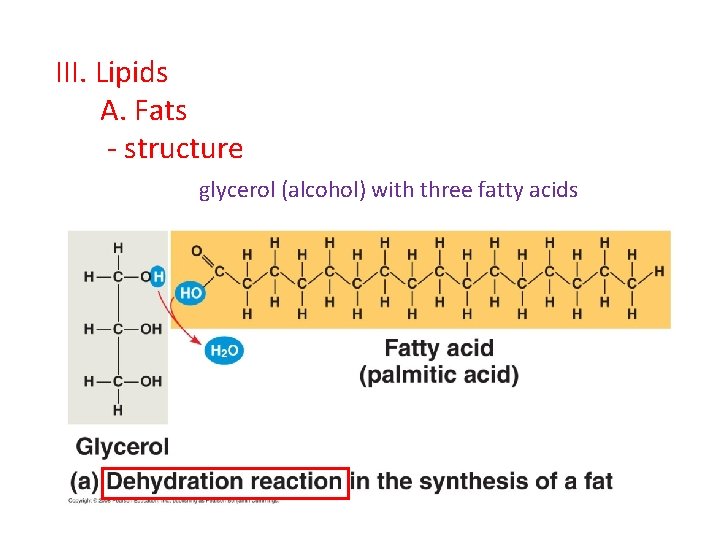
III. Lipids A. Fats - structure glycerol (alcohol) with three fatty acids
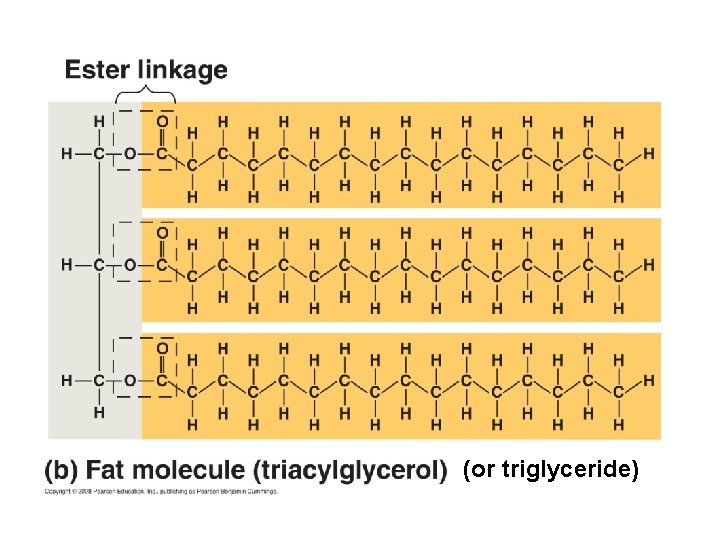
(or triglyceride)
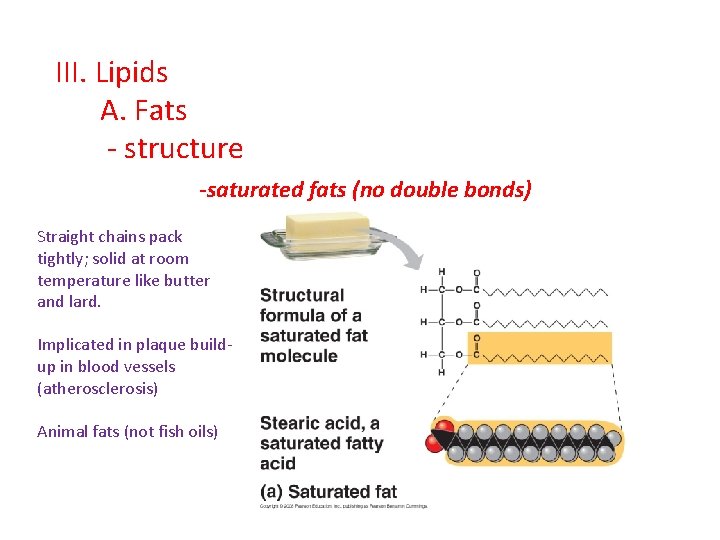
III. Lipids A. Fats - structure -saturated fats (no double bonds) Straight chains pack tightly; solid at room temperature like butter and lard. Implicated in plaque buildup in blood vessels (atherosclerosis) Animal fats (not fish oils)
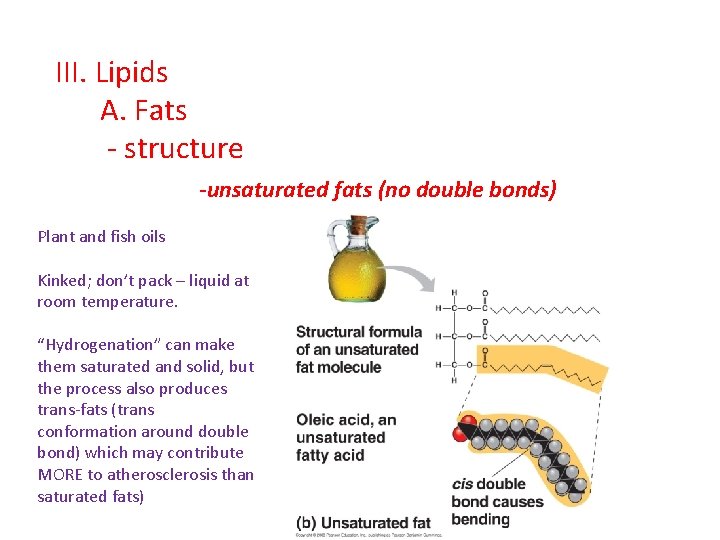
III. Lipids A. Fats - structure -unsaturated fats (no double bonds) Plant and fish oils Kinked; don’t pack – liquid at room temperature. “Hydrogenation” can make them saturated and solid, but the process also produces trans-fats (trans conformation around double bond) which may contribute MORE to atherosclerosis than saturated fats)

III. Lipids A. Fats - structure - functions - long term energy storage (dense) not vital in immobile organisms (mature plants), so it is metabolically easier to store energy as starch. But in seeds and animals (mobile), there is selective value to packing energy efficiently, in a small space. In animals, fat is stored in adipose cells

III. Lipids A. Fats - structure - functions - long term energy storage (dense) - insulation (subcutaneous fat) - cushioning
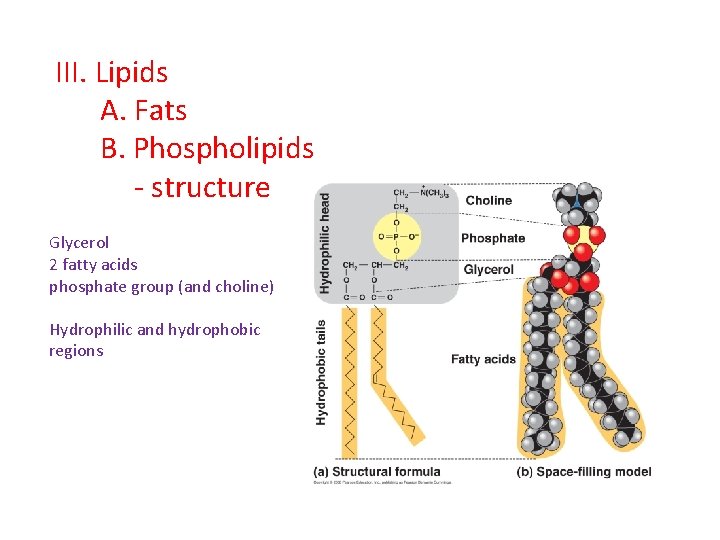
III. Lipids A. Fats B. Phospholipids - structure Glycerol 2 fatty acids phosphate group (and choline) Hydrophilic and hydrophobic regions
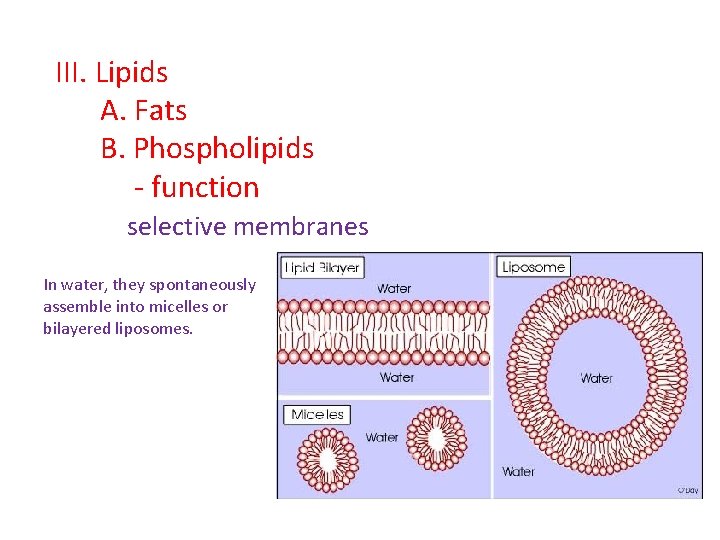
III. Lipids A. Fats B. Phospholipids - function selective membranes In water, they spontaneously assemble into micelles or bilayered liposomes.
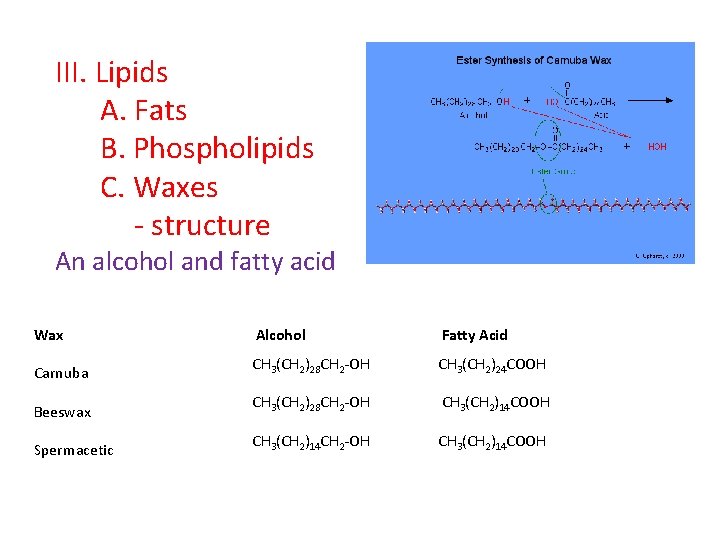
III. Lipids A. Fats B. Phospholipids C. Waxes - structure An alcohol and fatty acid Wax Alcohol Fatty Acid Carnuba CH 3(CH 2)28 CH 2 -OH CH 3(CH 2)24 COOH Beeswax CH 3(CH 2)28 CH 2 -OH CH 3(CH 2)14 COOH Spermacetic CH 3(CH 2)14 CH 2 -OH CH 3(CH 2)14 COOH

III. Lipids A. Fats B. Phospholipids C. Waxes - structure - function Retard the flow of water (plant waxes) Structural (beeswax in honeycomb) Signals – waxes on the exoskeleton can signal an insect’s identity and sexual receptivity.

III. Lipids A. Fats B. Phospholipids C. Waxes D. Steroids - structure typically a four-ring structure with side groups cholesterol and its hormone derivatives
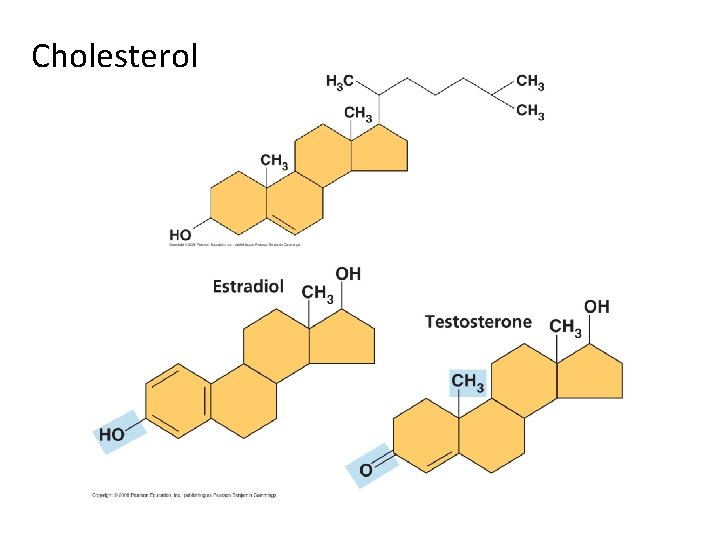
Cholesterol
Biologically Important Molecules I. III. IV. Water Carbohydrates Lipids Proteins
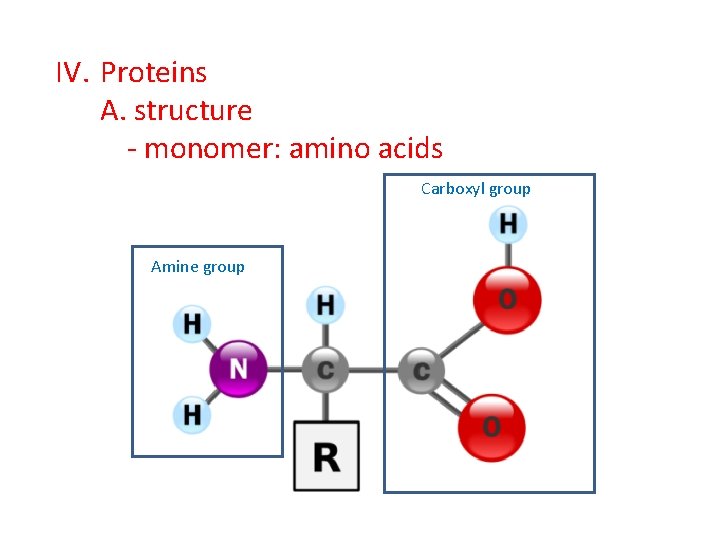
IV. Proteins A. structure - monomer: amino acids Carboxyl group Amine group
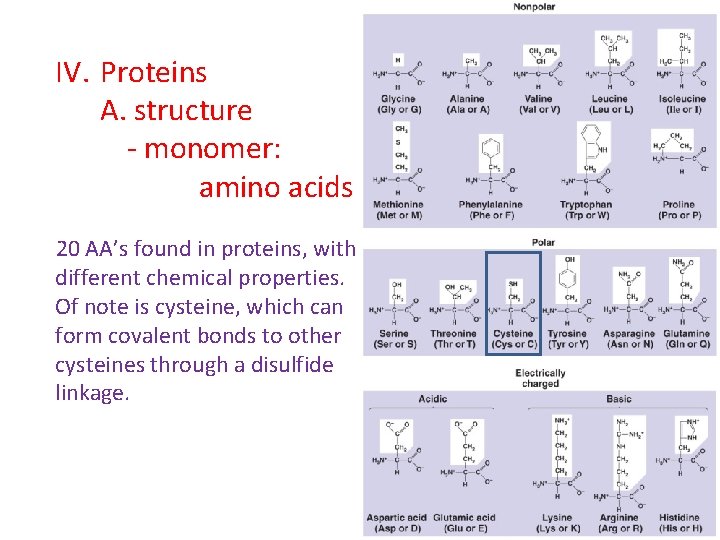
IV. Proteins A. structure - monomer: amino acids 20 AA’s found in proteins, with different chemical properties. Of note is cysteine, which can form covalent bonds to other cysteines through a disulfide linkage.
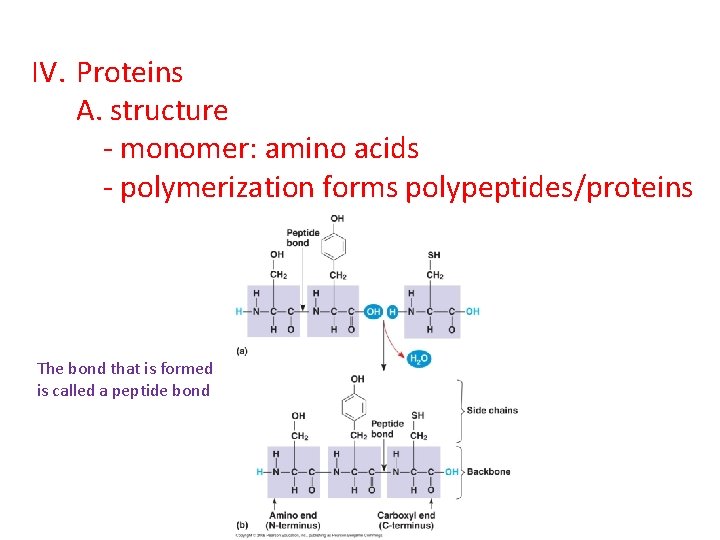
IV. Proteins A. structure - monomer: amino acids - polymerization forms polypeptides/proteins The bond that is formed is called a peptide bond
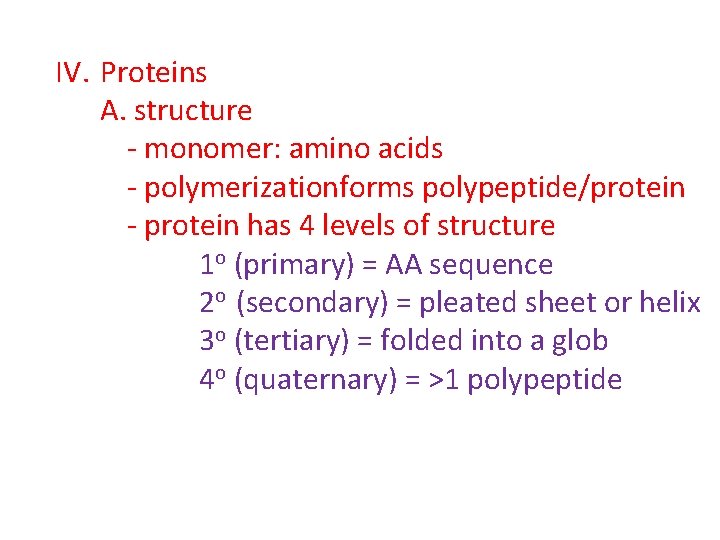
IV. Proteins A. structure - monomer: amino acids - polymerizationforms polypeptide/protein - protein has 4 levels of structure 1 o (primary) = AA sequence 2 o (secondary) = pleated sheet or helix 3 o (tertiary) = folded into a glob 4 o (quaternary) = >1 polypeptide
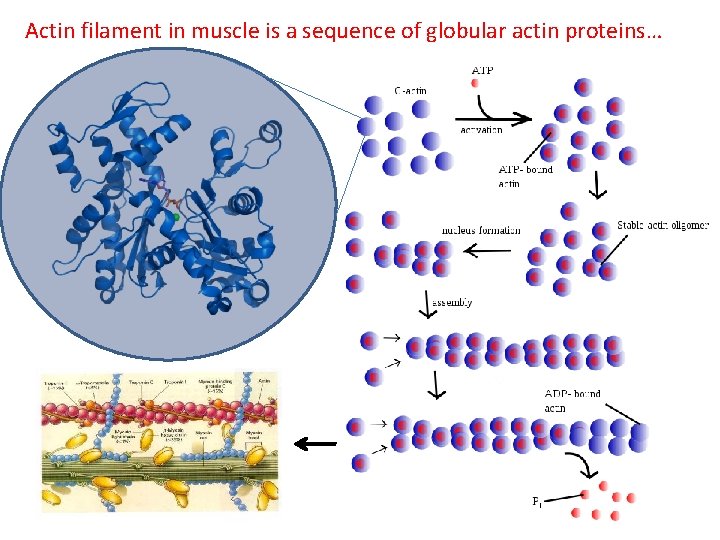
Actin filament in muscle is a sequence of globular actin proteins…
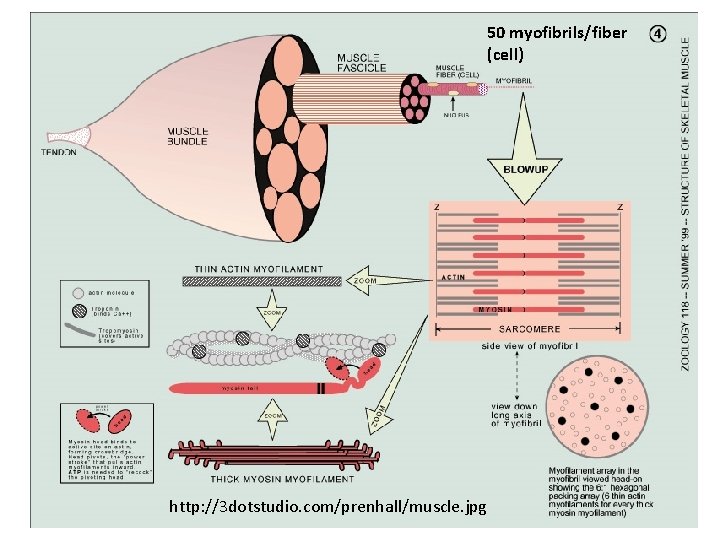
50 myofibrils/fiber (cell) http: //3 dotstudio. com/prenhall/muscle. jpg
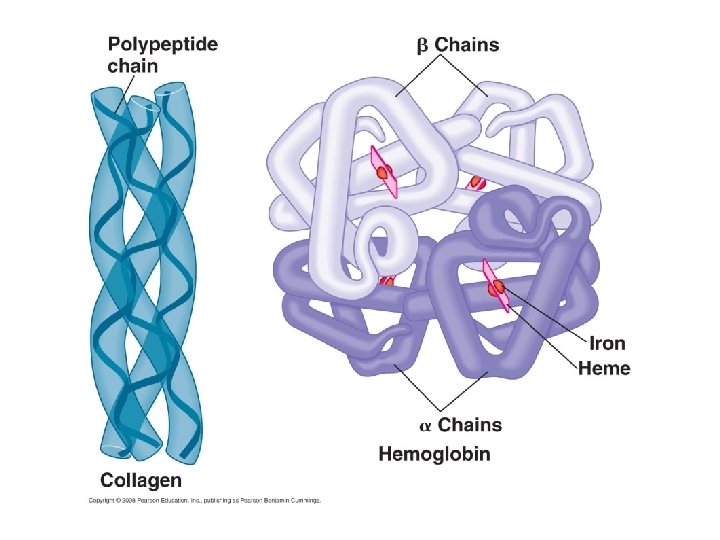
IV. Proteins A. structure B. functions! - catalysts (enzymes) - structural (actin/collagen/etc. ) - transport (hemoglobin, cell membrane) - immunity (antibodies) - cell signaling (surface antigens)
IV. Proteins A. structure B. functions! C. designer molecules If protein function is ultimately determined by AA sequence, why can’t we sequence a protein and then synthesize it? Folding is critical to function, and this is difficult to predict because it is often catalyzed by other molecules called chaparones By analyzing large numbers of protein sequences and structures, correlations between “functional motifs” and particular sequences are resolved.
- Slides: 35