Biological Science Sixth Edition Chapter 4 Nucleic Acids

Biological Science Sixth Edition Chapter 4 Nucleic Acids and the RNA World Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
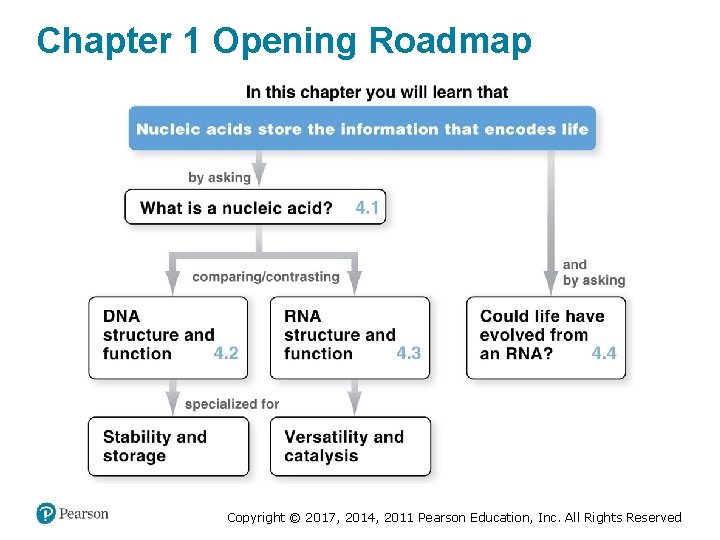
Chapter 1 Opening Roadmap Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
Introduction • Deoxyribonucelic acid (DNA) stores genetic information in modern cells • Most researchers think ribonucleic acid (RNA) was the first life molecule – Called the RNA world hypothesis – Proposes that RNA both stored genetic information and catalyzed its own replication • Once self-replicating molecules evolved, chemical evolution gave way to biological evolution Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
What Is a Nucleic Acid? (1 of 3) • A nucleic acid is a polymer of nucleotide monomers • Three components of a nucleotide: 1. A phosphate group 2. A five-carbon sugar 3. A nitrogenous (nitrogen-containing) base • Both the phosphate group and the nitrogenous base are bonded to the sugar molecule Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
What Is a Nucleic Acid? (2 of 3) • Ribonucleotides are the monomers of RNA – The sugar is ribose – Has an –OH group bonded to the 2′ carbon • Deoxyribonucleotides are the monomers of DNA – The sugar is deoxyribose (deoxy = “lacking oxygen”) – Has an H instead at the 2′ carbon • Both of these sugars have an –OH group bonded to the 3′ carbon Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
What Is a Nucleic Acid? (3 of 3) • There are two groups of nitrogenous bases: 1. Purines—contain nine atoms in their two rings § Adenine (A) § Guanine (G) 2. Pyrimidines—contain six atoms in their one ring § Cytosine (C) § Uracil (U)—found only in ribonucleotides § Thymine (T)—found only in deoxyribonucleotides Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
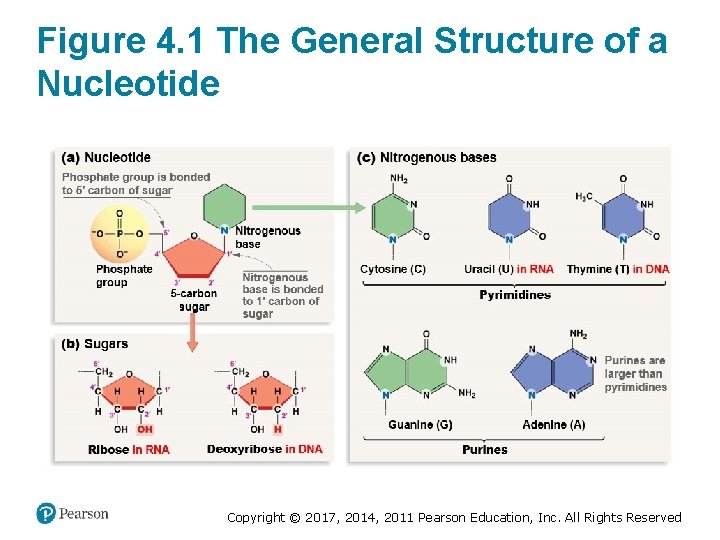
Figure 4. 1 The General Structure of a Nucleotide Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
Could Chemical Evolution Produce Nucleotides? • Simulations of chemical evolution – Have not yet produced nucleotides – Sugars and nitrogenous bases are easily made • Minerals in deep-sea vents preferentially bind to ribose – Produces a high concentration of ribose Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
Nucleotides Polymerize to Form Nucleic Acids • Nucleic acids form when nucleotides polymerize via condensation reactions • Phosphodiester linkage (bond) occurs between – The phosphate group on the 5′ carbon of one nucleotide – And the –OH group on the 3′ carbon of another • Two types of nucleotides are involved 1. Ribonucleotides polymerize to form RNA 2. Deoxyribonucleotides polymerize to form DNA Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
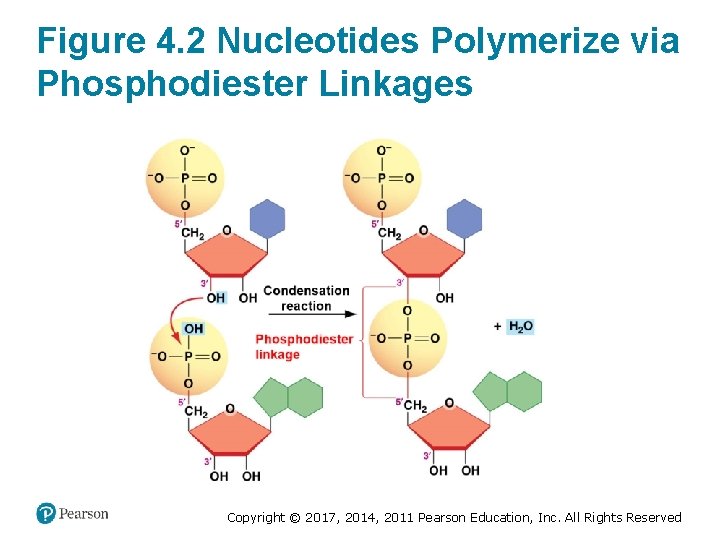
Figure 4. 2 Nucleotides Polymerize via Phosphodiester Linkages Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
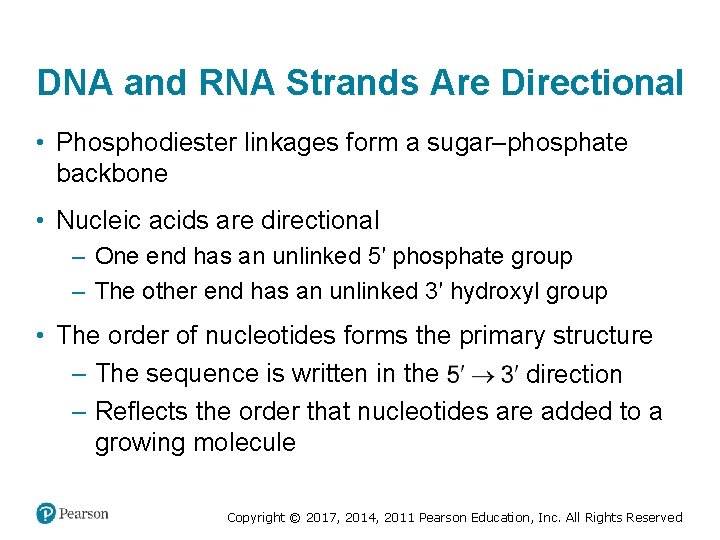
DNA and RNA Strands Are Directional • Phosphodiester linkages form a sugar–phosphate backbone • Nucleic acids are directional – One end has an unlinked 5′ phosphate group – The other end has an unlinked 3′ hydroxyl group • The order of nucleotides forms the primary structure – The sequence is written in the direction – Reflects the order that nucleotides are added to a growing molecule Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
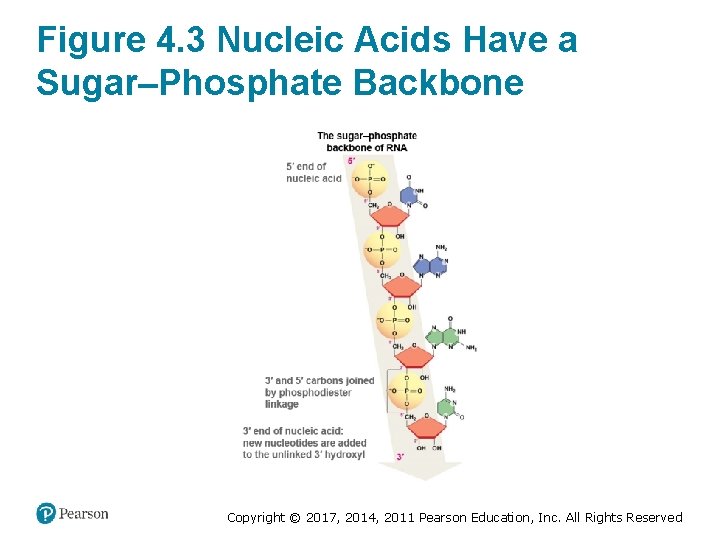
Figure 4. 3 Nucleic Acids Have a Sugar–Phosphate Backbone Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
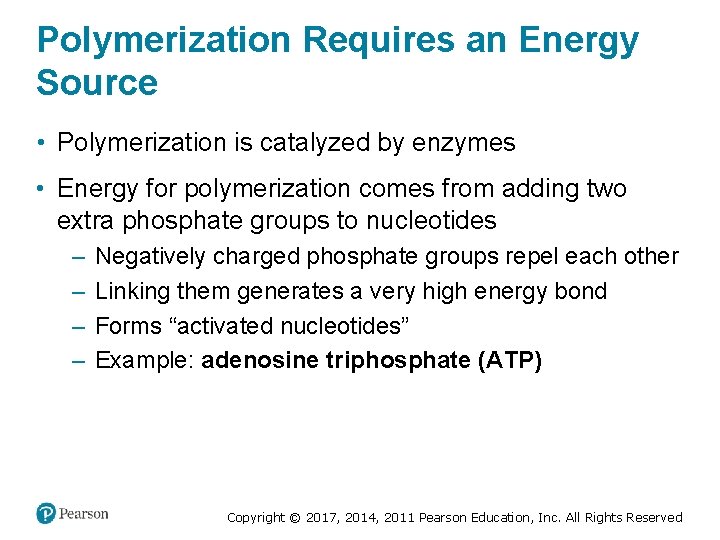
Polymerization Requires an Energy Source • Polymerization is catalyzed by enzymes • Energy for polymerization comes from adding two extra phosphate groups to nucleotides – – Negatively charged phosphate groups repel each other Linking them generates a very high energy bond Forms “activated nucleotides” Example: adenosine triphosphate (ATP) Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
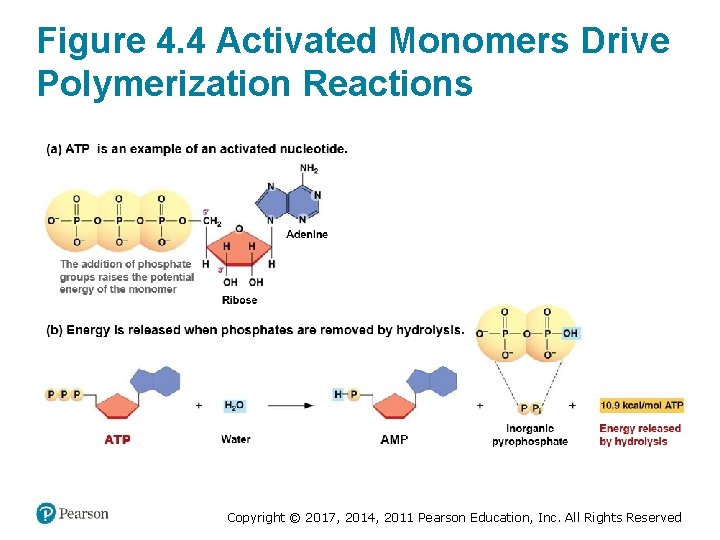
Figure 4. 4 Activated Monomers Drive Polymerization Reactions Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
What Is DNA’s Secondary Structure? • Early data provided clues to DNA secondary structure – Chemists knew the structure of nucleotides and that DNA has a sugar–phosphate backbone – Chargaff established that § # of purines = # of pyrimidines § Equal number of T’s and A’s; equal number of C’s and G’s – Franklin and Wilkins used X-ray crystallography to measure distances between atoms in DNA § Predicted a helical structure Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
DNA Strands Form an Antiparallel Double Helix (1 of 2) • James Watson and Francis Crick determined that – Two strands are held together by hydrogen bonds between pyramidines and purines – Complementary base pairing (Watson–Crick pairing) occurs between A and T, C and G – DNA strands are antiparallel § One strand runs the other runs – DNA strands form a double helix § The sugar–phosphate backbone faces the exterior § Nitrogenous base pairs face the interior Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
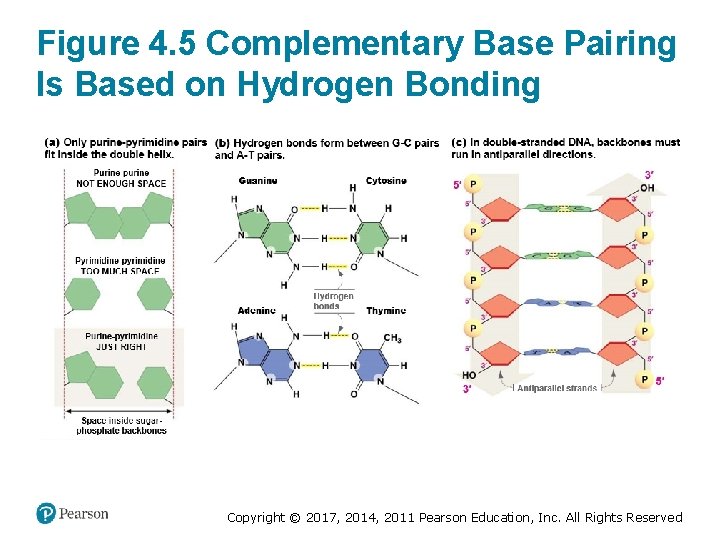
Figure 4. 5 Complementary Base Pairing Is Based on Hydrogen Bonding Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
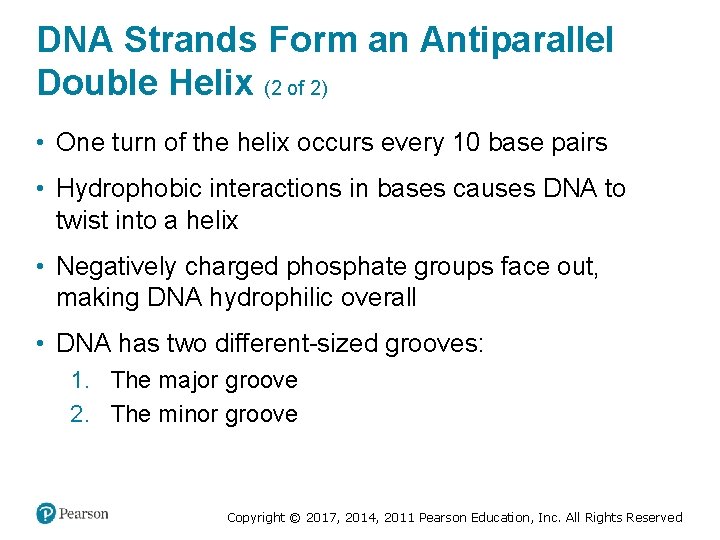
DNA Strands Form an Antiparallel Double Helix (2 of 2) • One turn of the helix occurs every 10 base pairs • Hydrophobic interactions in bases causes DNA to twist into a helix • Negatively charged phosphate groups face out, making DNA hydrophilic overall • DNA has two different-sized grooves: 1. The major groove 2. The minor groove Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
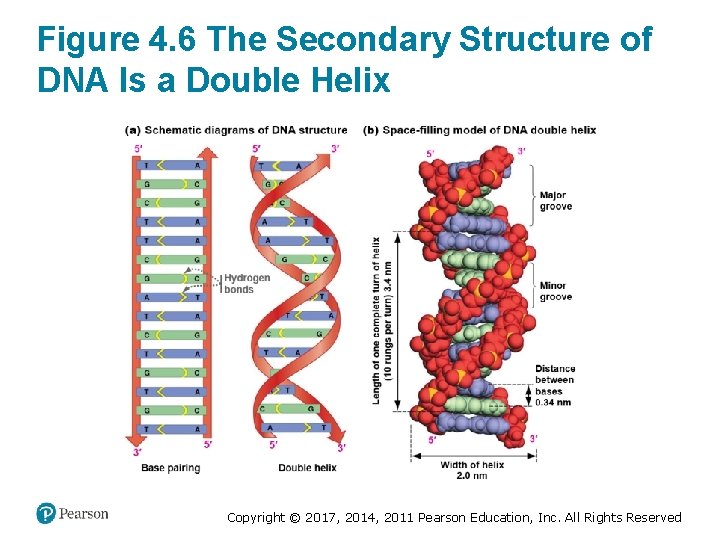
Figure 4. 6 The Secondary Structure of DNA Is a Double Helix Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
Cell Biology Video: Stick Model of DNA Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
Cell Biology Video: Surface Model of DNA Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
The Tertiary Structure of DNA • DNA forms more compact three-dimensional structures in cells – When it is wound too tightly or loosely, it twists to form supercoils – It wraps around proteins • Compacting DNA – Allows discrete units for cell division – Helps DNA fit inside the nucleus – Contribute to its function Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
DNA Functions as an Information Molecule (1 of 3) • DNA can store and transmit biological information • DNA carries the information required for the organism’s growth and reproduction • The language of nucleic acids is contained in the sequence of the bases – Nitrogenous bases function like letters in an alphabet – The sequence of bases has meaning, like the order of letters in a word Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
DNA Functions as an Information Molecule (2 of 3) • DNA’s primary structure serves as a template for the synthesis of a complementary strand – Contains the information required for a copy of itself to be made – Requires enzymes in modern cells Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
DNA Functions as an Information Molecule (3 of 3) • DNA replication has three steps: Step 1: The two strands are separated by breaking the hydrogen bonds with heat or enzymes Step 2: Free deoxyribonucleotides hydrogen—bond complementary bases on the original strand of DNA, also called the template strand – Phosphodiester linkages form to create a new strand, called the complementary strand Step 3: Complementary base pairing allows each strand to be copied exactly – Produces two identical daughter molecules Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
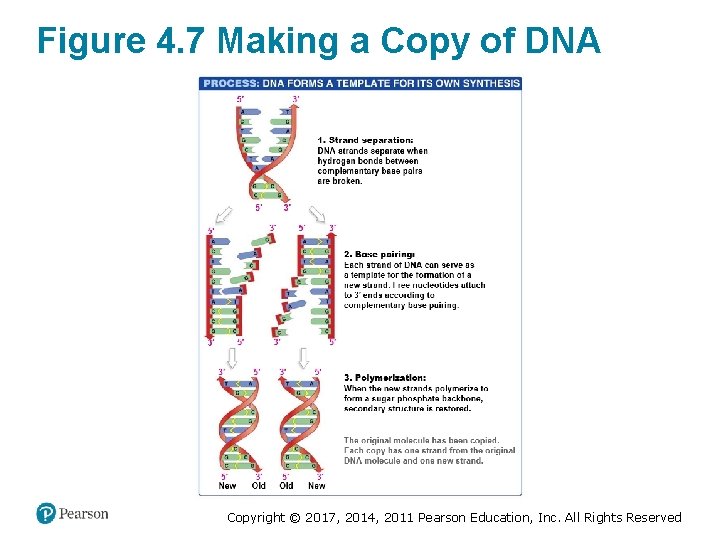
Figure 4. 7 Making a Copy of DNA Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
The DNA Double Helix Is a Stable Structure • DNA is very stable – It is resistant to chemical degradation – Makes it a reliable store for genetic information • Stable molecules such as DNA make poor catalysts – DNA has never been observed to catalyze a reaction • Biologists think that the first life-form was made of RNA Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
Structurally, RNA Differs from DNA • RNA (like DNA) has a primary structure consisting of – A sugar–phosphate backbone formed by phosphodiester linkages – Four types of nitrogenous bases extending from it • The primary structure of RNA differs from DNA: 1. RNA contains ribose instead of deoxyribose § 2′ −OH group on ribose is more reactive than −H § RNA is much less stable than DNA 2. RNA contains uracil instead of thymine Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
Secondary Structure • RNA’s secondary structure results from complementary base pairing: A with U; G with C • The bases of RNA typically form hydrogen bonds with complementary bases on the same strand • The RNA strand folds over, forming a hairpin structure – The bases on one side of the fold. . . – Align with an antiparallel RNA segment on the other side of the fold Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
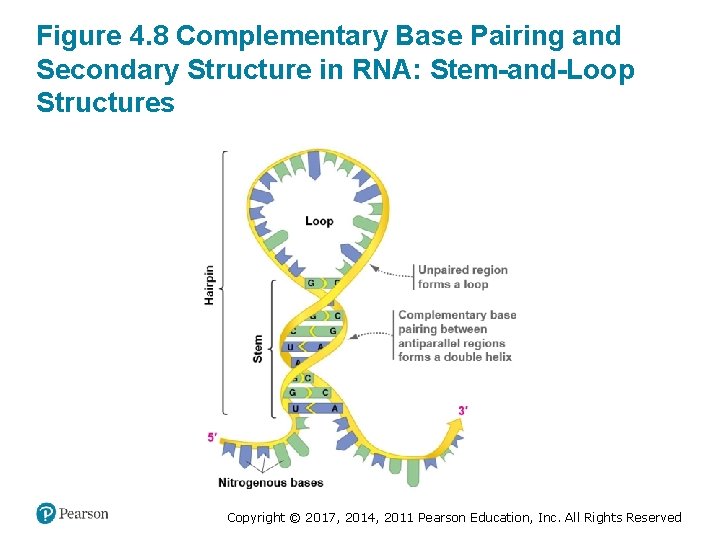
Figure 4. 8 Complementary Base Pairing and Secondary Structure in RNA: Stem-and-Loop Structures Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
RNA Can Function as a Catalytic Molecule (1 of 2) • RNA molecules can also have tertiary structure – Forms when secondary structures fold into more complex shapes • RNA is much more diverse in size, shape, and reactivity than DNA • RNA is highly versatile – An information-containing molecule – Capable of self-replication – Capable of catalyzing reactions: ribozymes Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
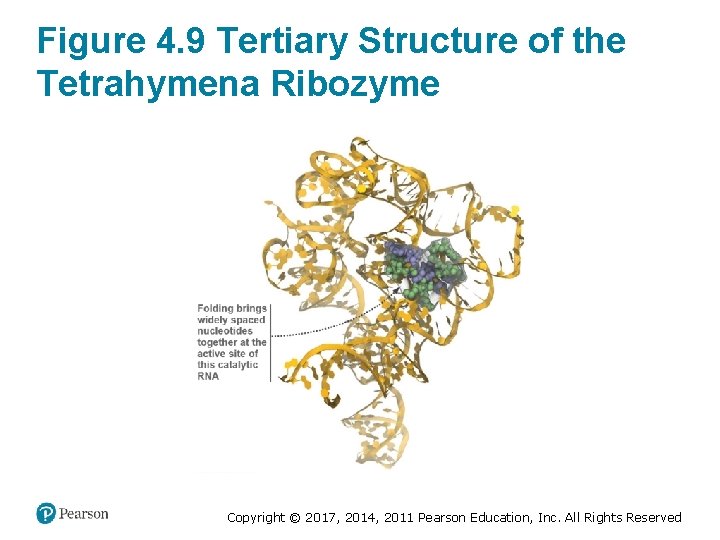
Figure 4. 9 Tertiary Structure of the Tetrahymena Ribozyme Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
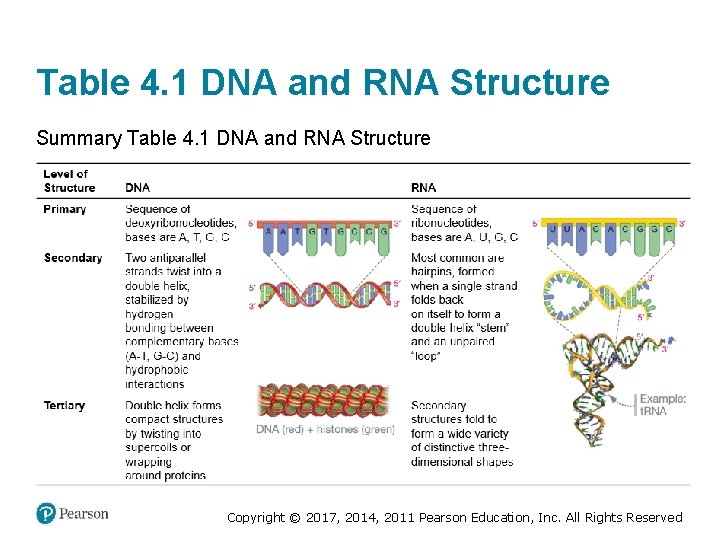
Table 4. 1 DNA and RNA Structure Summary Table 4. 1 DNA and RNA Structure Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
RNA Can Function as a Catalytic Molecule (2 of 2) • RNA is capable of self-replication – Information stored in RNA can be used to make copies via complementary base pairing – Must pass through double-stranded intermediates – Process occurs today in some viruses Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
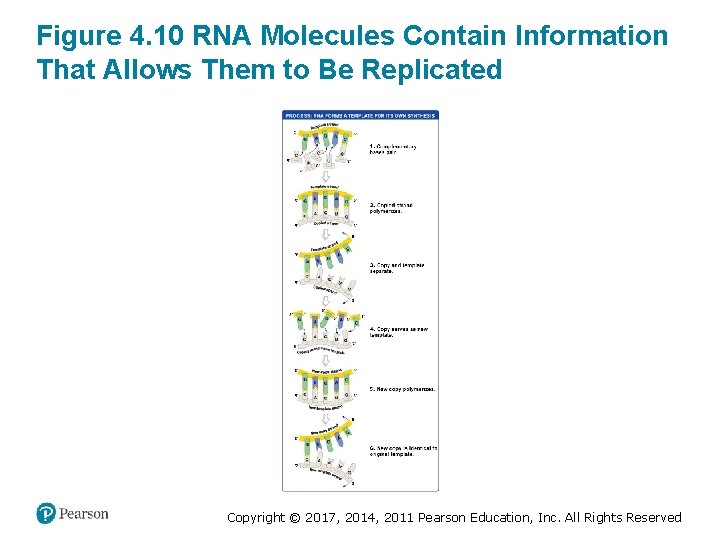
Figure 4. 10 RNA Molecules Contain Information That Allows Them to Be Replicated Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
In Search of the First Life-Form • The theory of chemical evolution – Life began as a naked self-replicator – A molecule in solution not enclosed in a membrane • To copy itself, the first living molecule had to – Provide a template that could be copied – Polymerize monomers into a copy of that template • RNA is capable of both processes – Most researchers propose the first life-form was made of RNA Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
The RNA World May Have Sparked the Evolution of Life • Most modern ribozymes aid protein production – If removed, proteins could not be made – Therefore, RNA probably preceded proteins • Evolution of protein enzymes occurred later • Three characteristics of life are accounted for: 1. Information processing 2. Replication of hereditary information 3. Evolution by random changes in nucleic acids Copyright © 2017, 2014, 2011 Pearson Education, Inc. All Rights Reserved
- Slides: 37