BIOLOGICAL SCIENCE Freeman Quillin Allison 3 Protein Structure

BIOLOGICAL SCIENCE Freeman Quillin Allison 3 Protein Structure and Function Lecture Presentation by Cindy S. Malone, Ph. D, California State University Northridge © 2014 Pearson Education, Inc. FIFTH EDITION
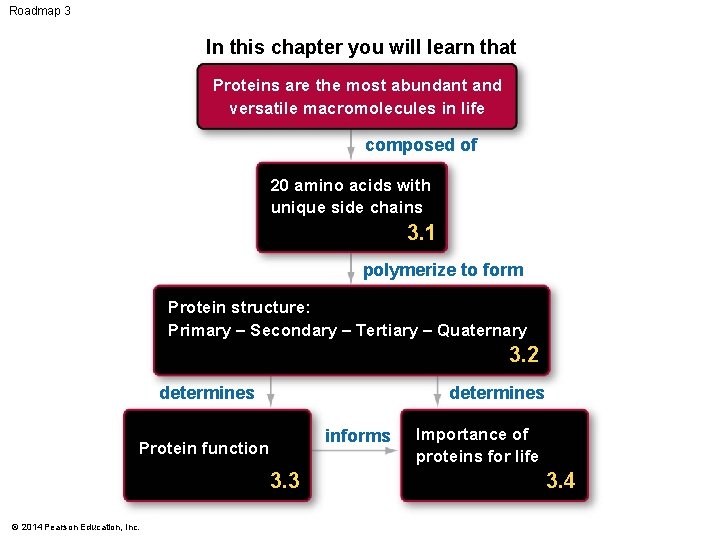
Roadmap 3 In this chapter you will learn that Proteins are the most abundant and versatile macromolecules in life composed of 20 amino acids with unique side chains 3. 1 polymerize to form Protein structure: Primary – Secondary – Tertiary – Quaternary 3. 2 determines informs Protein function 3. 3 © 2014 Pearson Education, Inc. Importance of proteins for life 3. 4
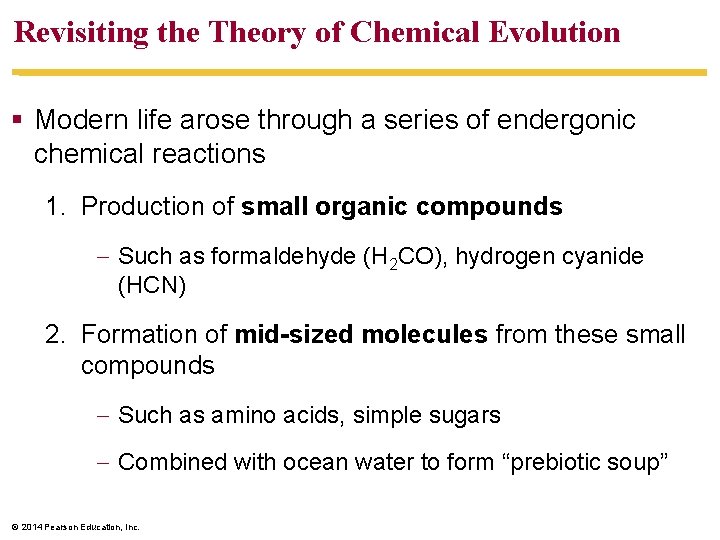
Revisiting the Theory of Chemical Evolution Modern life arose through a series of endergonic chemical reactions 1. Production of small organic compounds Such as formaldehyde (H 2 CO), hydrogen cyanide (HCN) 2. Formation of mid-sized molecules from these small compounds Such as amino acids, simple sugars Combined with ocean water to form “prebiotic soup” © 2014 Pearson Education, Inc.
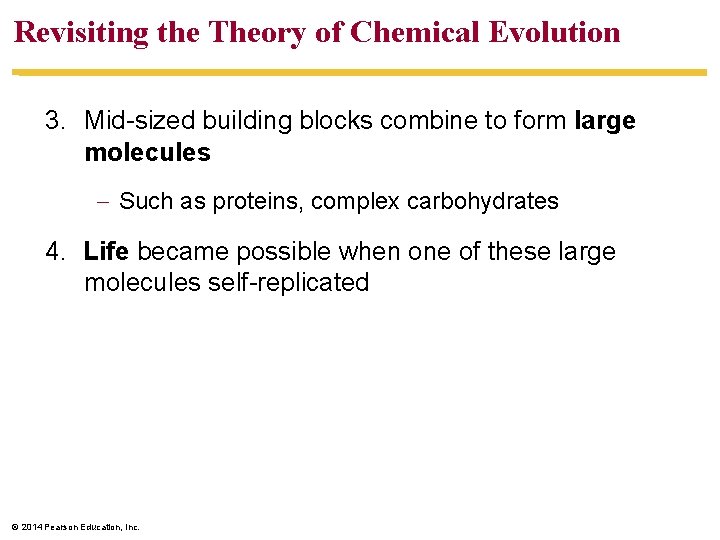
Revisiting the Theory of Chemical Evolution 3. Mid-sized building blocks combine to form large molecules Such as proteins, complex carbohydrates 4. Life became possible when one of these large molecules self-replicated © 2014 Pearson Education, Inc.
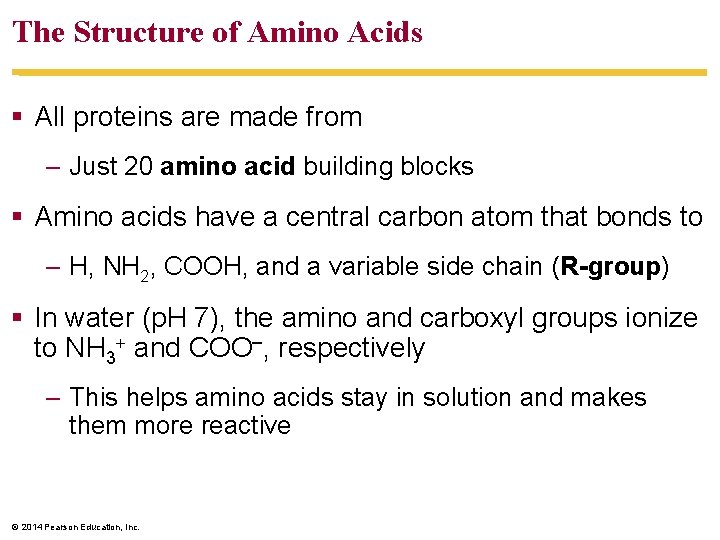
The Structure of Amino Acids All proteins are made from – Just 20 amino acid building blocks Amino acids have a central carbon atom that bonds to – H, NH 2, COOH, and a variable side chain (R-group) In water (p. H 7), the amino and carboxyl groups ionize to NH 3 and COO–, respectively – This helps amino acids stay in solution and makes them more reactive © 2014 Pearson Education, Inc.
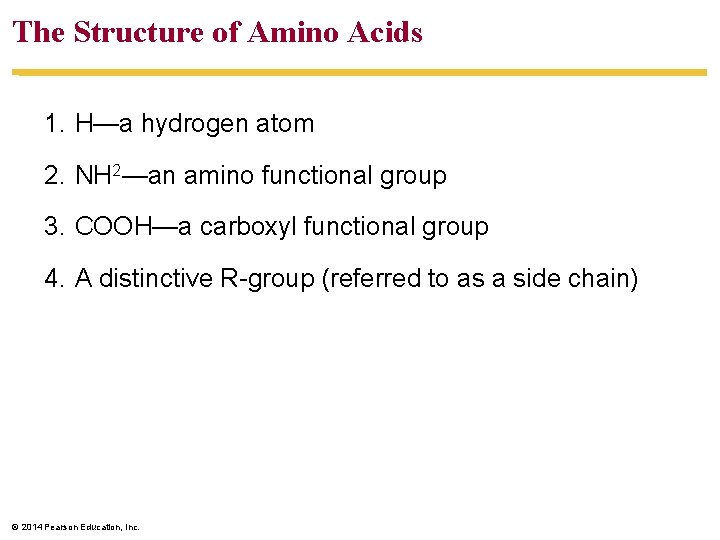
The Structure of Amino Acids 1. H—a hydrogen atom 2. NH 2—an amino functional group 3. COOH—a carboxyl functional group 4. A distinctive R-group (referred to as a side chain) © 2014 Pearson Education, Inc.
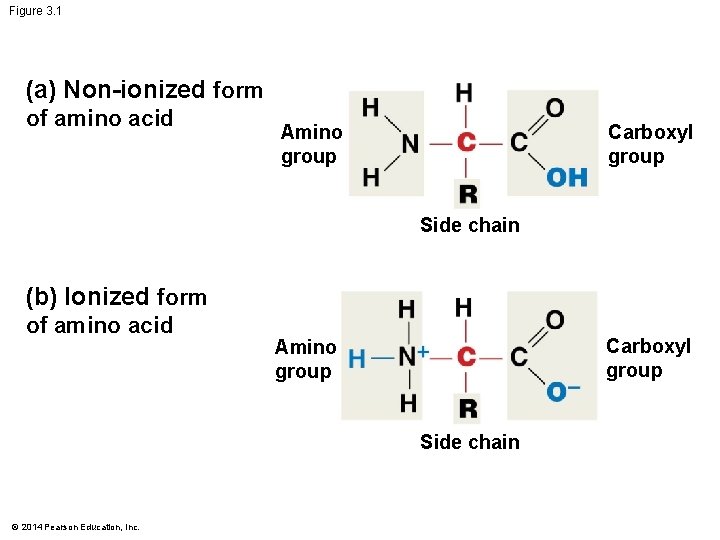
Figure 3. 1 (a) Non-ionized form of amino acid Amino group Carboxyl group Side chain (b) Ionized form of amino acid Carboxyl group Amino group Side chain © 2014 Pearson Education, Inc.

The Nature of Side Chains The 20 amino acids differ only – In the unique R-group attached to the central carbon The properties of amino acids vary – Because their R-groups vary Amino acid side chains distinguish the different amino acids and can be grouped into four general types: – – Acidic Basic Uncharged polar Nonpolar © 2014 Pearson Education, Inc.
The Nature of Side Chains If given a structural formula for an amino acid – Determine the amino acid type by asking three questions: 1. Does the side chain have a negative charge? If so, it has lost a proton, so it must be acidic 2. Does the side chain have a positive charge? If so, it has taken on a proton, so it must be basic © 2014 Pearson Education, Inc.
The Nature of Side Chains 3. If side chain is uncharged, does it have an oxygen atom? If so, the highly electronegative oxygen will result in a polar covalent bond and thus is uncharged polar If the answers to all three questions are no – Then you are looking at a nonpolar amino acid © 2014 Pearson Education, Inc.
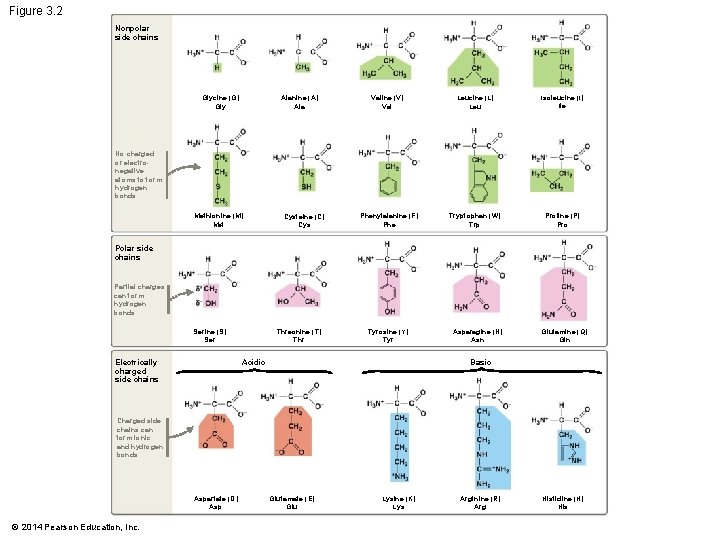
Figure 3. 2 Nonpolar side chains Glycine (G) Gly Alanine (A) Ala Valine (V) Val Leucine (L) Leu Isoleucine (I) Ile Phenylalanine (F) Phe Tryptophan (W) Trp Proline (P) Pro No charged or electronegative atoms to form hydrogen bonds Methionine (M) Met Cysteine (C) Cys Polar side chains Partial charges can form hydrogen bonds Serine (S) Ser Electrically charged side chains Threonine (T) Thr Tyrosine (Y) Tyr Acidic Asparagine (N) Asn Glutamine (Q) Gln Basic Charged side chains can form ionic and hydrogen bonds Aspartate (D) Asp © 2014 Pearson Education, Inc. Glutamate (E) Glu Lysine (K) Lys Arginine (R) Arg Histidine (H) His

Functional Groups Affect Reactivity R-groups differ in their size, shape, reactivity, and interactions with water – Nonpolar R-groups: hydrophobic Do not form hydrogen bonds Coalesce in water Lack charged or highly electronegative atoms capable of forming hydrogen bonds with water – Polar R-groups: hydrophilic Form hydrogen bonds Readily dissolve in water © 2014 Pearson Education, Inc.
Functional Groups Affect Reactivity Several amino acid side chains contain hydroxyl, amino, carboxyl, or sulfhydryl functional groups These groups are more chemically reactive than those with side chains composed of only carbon and hydrogen atoms © 2014 Pearson Education, Inc.
Monomers and Polymers Many mid-sized molecules – Such as amino acids and nucleotides – Are individual units called monomers They link together (polymerize) to form polymers – Such as proteins and nucleic acids Macromolecules are – Very large polymers made up of many monomers linked together Proteins are – Macromolecules consisting of amino acid monomers linked through chemical bonds © 2014 Pearson Education, Inc.
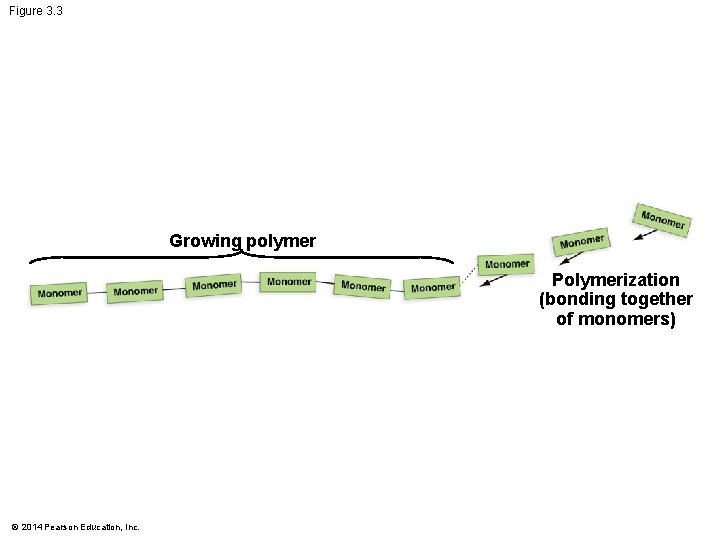
Figure 3. 3 Growing polymer Polymerization (bonding together of monomers) © 2014 Pearson Education, Inc.
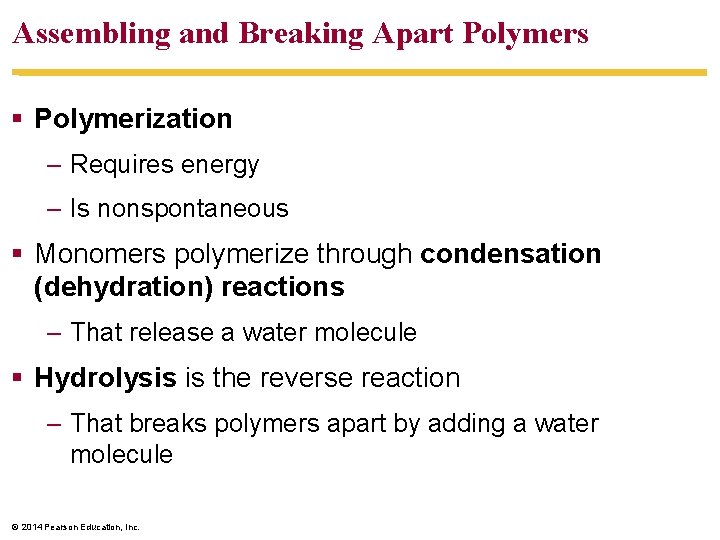
Assembling and Breaking Apart Polymers Polymerization – Requires energy – Is nonspontaneous Monomers polymerize through condensation (dehydration) reactions – That release a water molecule Hydrolysis is the reverse reaction – That breaks polymers apart by adding a water molecule © 2014 Pearson Education, Inc.
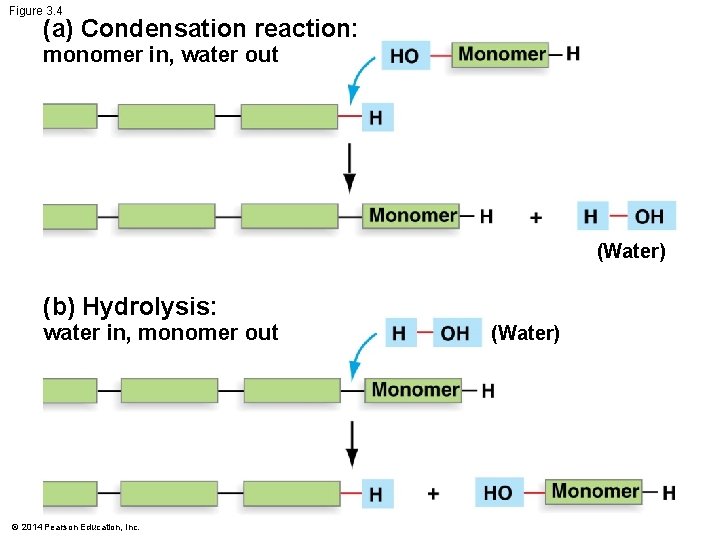
Figure 3. 4 (a) Condensation reaction: monomer in, water out (Water) (b) Hydrolysis: water in, monomer out © 2014 Pearson Education, Inc. (Water)
The Peptide Bond Peptide bond – Condensation reactions Bond the carboxyl group of one amino acid To the amino group of another Polypeptide – A chain of amino acids linked by peptide bonds – Oligopeptides (peptides) Polypeptides containing fewer than 50 amino acids – Proteins Polypeptides containing more than 50 amino acids © 2014 Pearson Education, Inc.
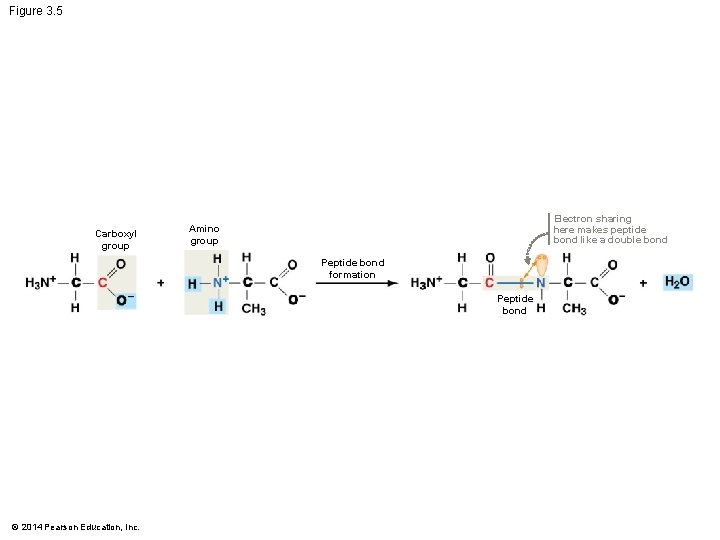
Figure 3. 5 Carboxyl group Electron sharing here makes peptide bond like a double bond Amino group Peptide bond formation Peptide bond © 2014 Pearson Education, Inc.
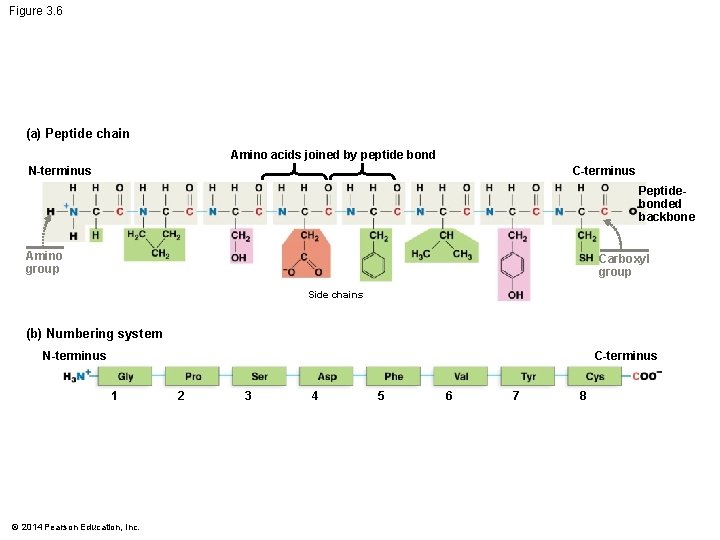
Figure 3. 6 (a) Peptide chain Amino acids joined by peptide bond N-terminus C-terminus Peptidebonded backbone Amino group Carboxyl group Side chains (b) Numbering system C-terminus N-terminus 1 © 2014 Pearson Education, Inc. 2 3 4 5 6 7 8
What Do Proteins Do? Proteins are crucial to most tasks required for cells to exist Catalysis – Enzymes speed up chemical reactions Defense – Antibodies and complement proteins attack pathogens Movement – Motor and contractile proteins move the cell or molecules within the cell © 2014 Pearson Education, Inc.
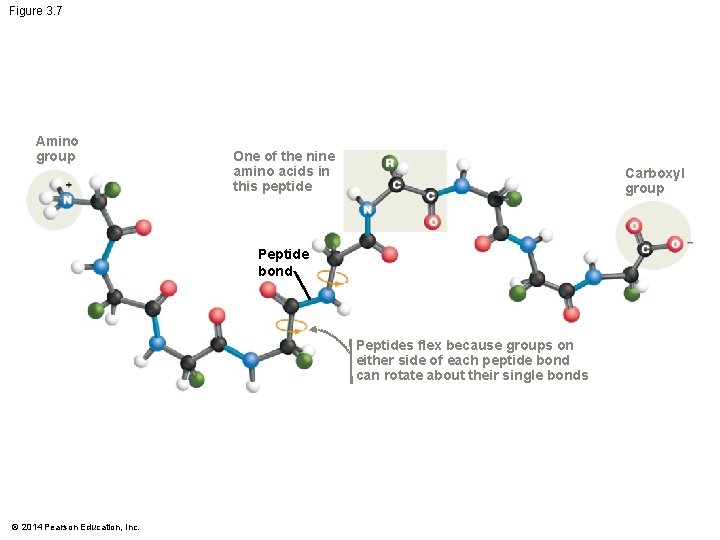
Figure 3. 7 Amino group One of the nine amino acids in this peptide Carboxyl group Peptide bond Peptides flex because groups on either side of each peptide bond can rotate about their single bonds © 2014 Pearson Education, Inc.
Polypeptide Characteristics Within the polypeptide, the peptide bonds form a backbone with three key characteristics: 1. R-group orientation Side chains can interact with each other or water 2. Directionality Free amino group, on the left, is called the N-terminus Free carboxyl group, on the right, is called the C-terminus 3. Flexibility Single bonds on either side of the peptide bond can rotate These bonds make the entire structure flexible © 2014 Pearson Education, Inc.
What Do Proteins Do? Signaling – Proteins convey signals between cells Structure – Structural proteins define cell shape and comprise body structures Transport – Transport proteins carry materials – Membrane proteins control molecular movement into and out of the cell © 2014 Pearson Education, Inc.
What Do Proteins Look Like? The unparalleled diversity of proteins in size, shape, and other aspects of structure is important because – Function follows from structure Proteins can serve diverse functions in cells because they are – Diverse in size and shape – Diverse in the chemical properties of their amino acids © 2014 Pearson Education, Inc.
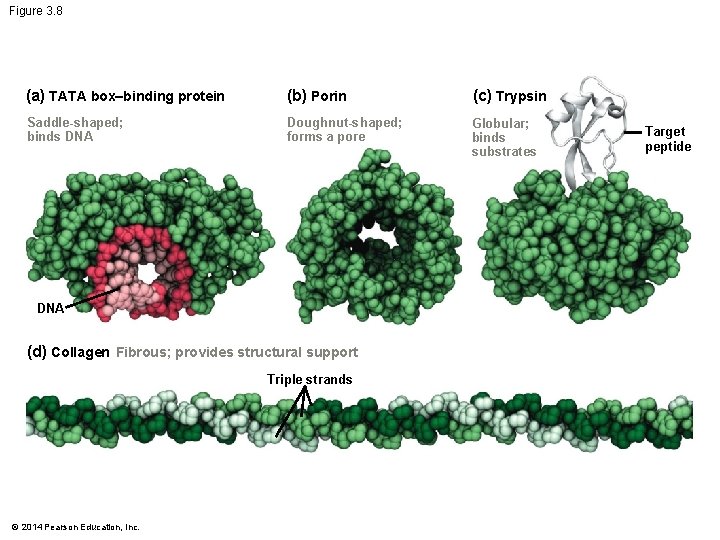
Figure 3. 8 (a) TATA box–binding protein (b) Porin (c) Trypsin Saddle-shaped; binds DNA Doughnut-shaped; forms a pore Globular; binds substrates DNA (d) Collagen Fibrous; provides structural support Triple strands © 2014 Pearson Education, Inc. Target peptide
What Do Proteins Look Like? All proteins have just four basic levels of structure: 1. Primary 2. Secondary 3. Tertiary 4. Quaternary © 2014 Pearson Education, Inc.
Primary Structure Protein primary structure is its unique sequence of amino acids The number of possible primary structures is practically limitless – 20 types of amino acids available – Lengths range from two amino acid residues to tens of thousands © 2014 Pearson Education, Inc.
Primary Structure Primary structure is fundamental to the higher levels of protein structure – Secondary, tertiary, and quaternary The amino acid R-groups affect a polypeptide’s properties and function – A single amino acid change can radically alter protein function © 2014 Pearson Education, Inc.
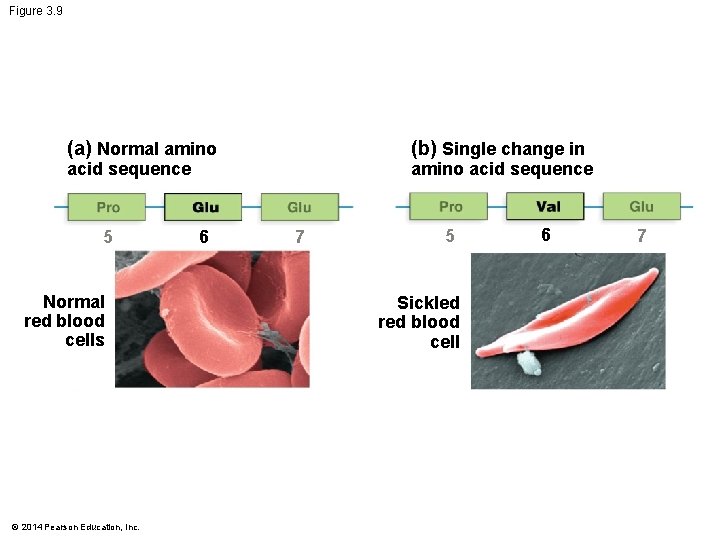
Figure 3. 9 (b) Single change in (a) Normal amino acid sequence 5 Normal red blood cells © 2014 Pearson Education, Inc. 6 7 5 Sickled red blood cell 6 7
Secondary Structure Protein’s secondary structure is formed by hydrogen bonds Hydrogen bonds occur between – The carbonyl group of one amino acid – And the amino group of another Hydrogen bonding between sections of the same backbone – Is possible only when a polypeptide bends in a way that puts C O and N–H groups close together, forming -helices -pleated sheets © 2014 Pearson Education, Inc.
Secondary Structure Secondary structure depends on the primary structure – Some amino acids are more likely to be involved in -helices – Others are likely to be involved in -pleated sheets © 2014 Pearson Education, Inc.
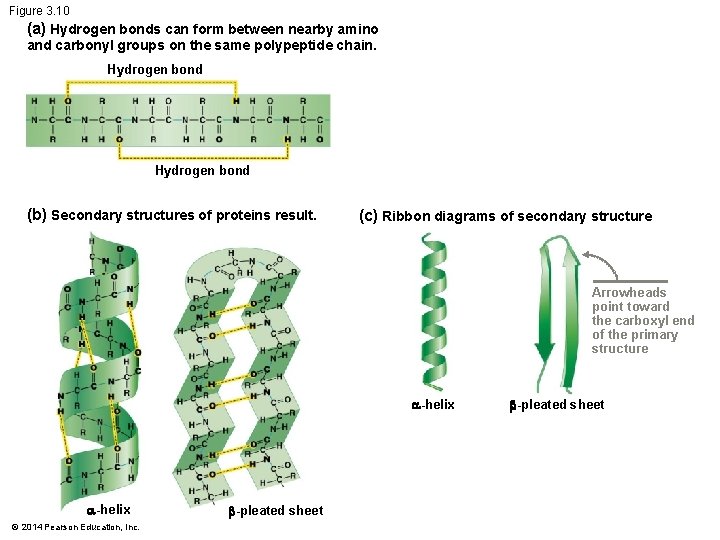
Figure 3. 10 (a) Hydrogen bonds can form between nearby amino and carbonyl groups on the same polypeptide chain. Hydrogen bond (b) Secondary structures of proteins result. (c) Ribbon diagrams of secondary structure Arrowheads point toward the carboxyl end of the primary structure -helix © 2014 Pearson Education, Inc. -pleated sheet
Tertiary Structure The tertiary structure of a polypeptide results from – Interactions between R-groups – Or between R-groups and the peptide backbone These contacts cause the backbone to bend and fold Bending and folding contribute to the distinctive threedimensional shape of the polypeptide © 2014 Pearson Education, Inc.
Tertiary Structure R-group interactions include – Hydrogen bonds – Hydrophobic interactions – Van der Waals interactions – Covalent disulfide bonds – Ionic bonds © 2014 Pearson Education, Inc.
R-group Interactions That Form Tertiary Structures Hydrogen bonds form – Between hydrogen atoms and the carbonyl group in the peptide-bonded backbone – Between hydrogen and negatively charged atoms in side chains Hydrophobic interactions within a protein increase stability of – Surrounding water molecules by increasing hydrogen bonding © 2014 Pearson Education, Inc.
R-group Interactions That Form Tertiary Structures van der Waals interactions are – Weak electrical interactions between hydrophobic side chains Covalent disulfide bonds form – Between sulfur-containing R-groups Ionic bonds form – Between groups that have full and opposing charges © 2014 Pearson Education, Inc.
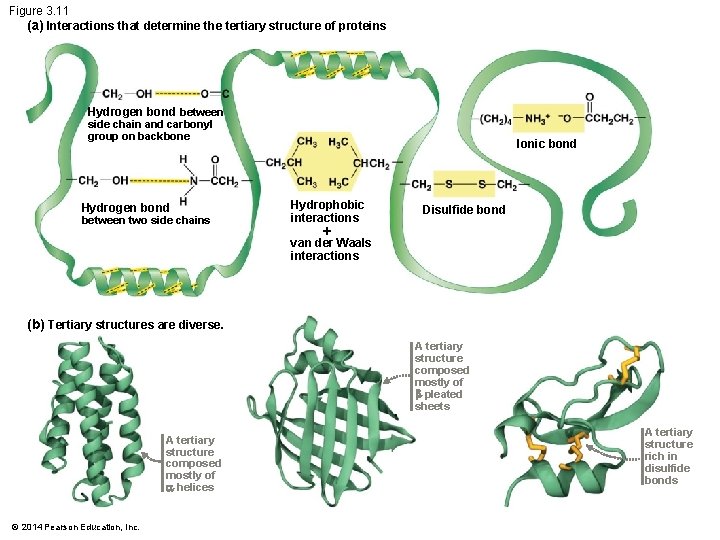
Figure 3. 11 (a) Interactions that determine the tertiary structure of proteins Hydrogen bond between side chain and carbonyl group on backbone Hydrogen bond between two side chains Ionic bond Hydrophobic interactions van der Waals interactions Disulfide bond (b) Tertiary structures are diverse. A tertiary structure composed mostly of -pleated sheets A tertiary structure composed mostly of -helices © 2014 Pearson Education, Inc. A tertiary structure rich in disulfide bonds
Quaternary Structure Many proteins contain several distinct polypeptide subunits that interact to form a single structure The bonding of two or more distinct polypeptide subunits produces quaternary structure © 2014 Pearson Education, Inc.
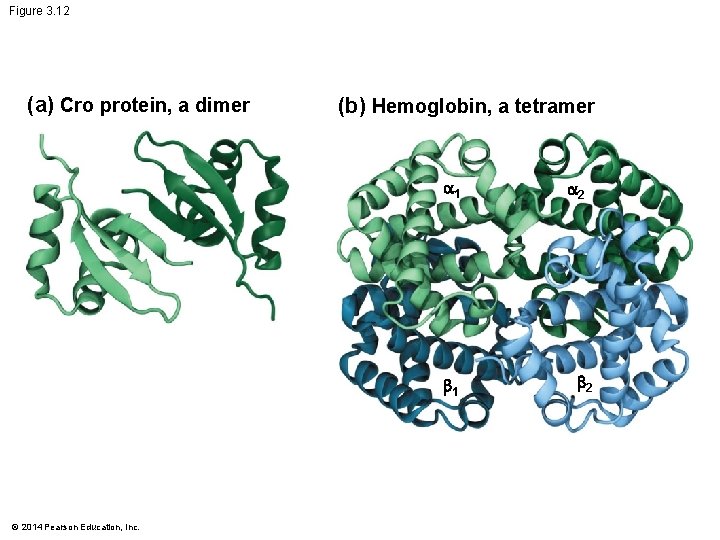
Figure 3. 12 (a) Cro protein, a dimer (b) Hemoglobin, a tetramer 1 1 © 2014 Pearson Education, Inc. 2 2
Summary of Protein Structure Protein structure is hierarchical – Quaternary structure is based on tertiary structure, which is based in part on secondary structure – All three of the higher-level structures are based on primary structure Combined effects of primary, secondary, tertiary, and sometimes quaternary structure – Allow for amazing diversity in protein form and function © 2014 Pearson Education, Inc.
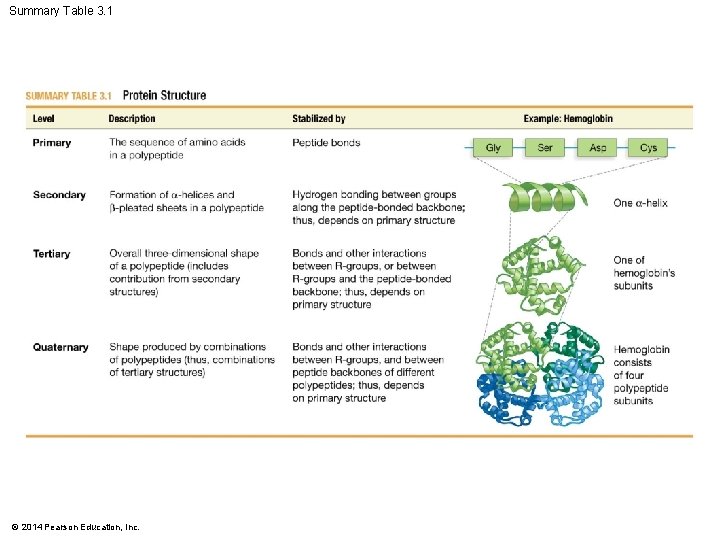
Summary Table 3. 1 © 2014 Pearson Education, Inc.
Folding and Function Protein folding is often spontaneous – Because of the hydrogen bonds and van der Waals interactions – That make the folded molecule more energetically stable than the unfolded molecule A denatured (unfolded) protein is unable to function normally Proteins called molecular chaperones help proteins fold correctly in cells © 2014 Pearson Education, Inc.
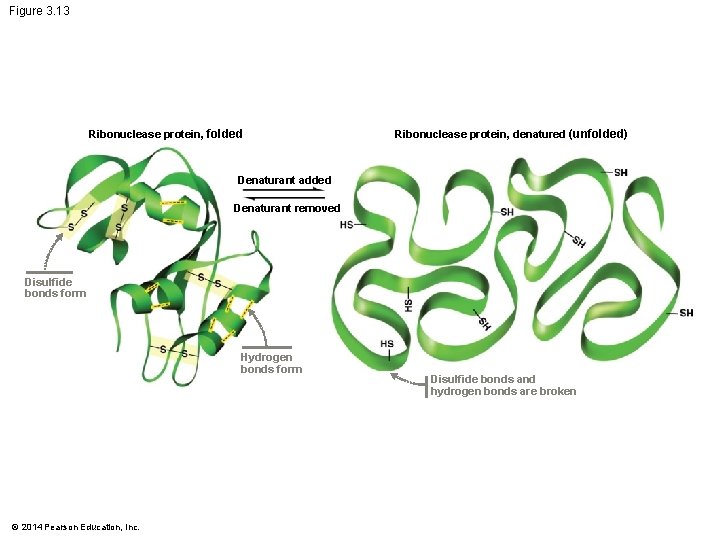
Figure 3. 13 Ribonuclease protein, folded Ribonuclease protein, denatured (unfolded) Denaturant added Denaturant removed Disulfide bonds form Hydrogen bonds form © 2014 Pearson Education, Inc. Disulfide bonds and hydrogen bonds are broken
Folding and Function Protein folding is often regulated Since the function of a protein is dependent on its shape – Controls when or where it is folded – Regulates the protein’s activity For example: – The inactive form of a protein has a disordered shape – When active protein is needed It folds into an ordered, active conformation © 2014 Pearson Education, Inc.
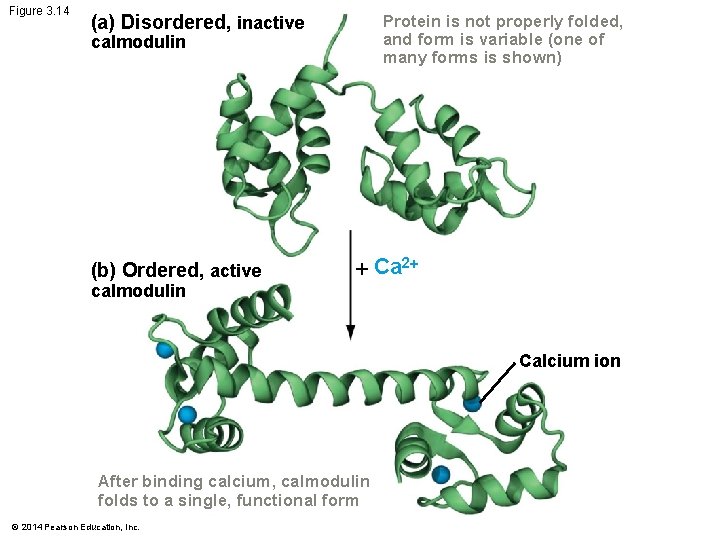
Figure 3. 14 (a) Disordered, inactive Protein is not properly folded, and form is variable (one of many forms is shown) calmodulin (b) Ordered, active Ca 2 calmodulin Calcium ion After binding calcium, calmodulin folds to a single, functional form © 2014 Pearson Education, Inc.
Prions and Protein Folding Misfolding can be “infectious” Prions are improperly folded forms of normal proteins – They are present in healthy individuals – Amino acid sequence does not differ from a normal protein – But its shape is radically different Prions can induce normal protein molecules to – Change their shape to the altered form © 2014 Pearson Education, Inc.
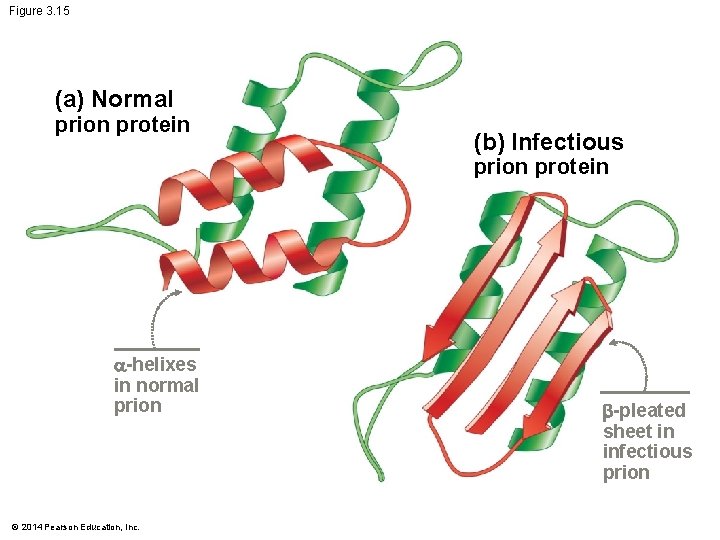
Figure 3. 15 (a) Normal prion protein (b) Infectious prion protein -helixes in normal prion © 2014 Pearson Education, Inc. -pleated sheet in infectious prion
What Do Proteins Do? Proteins are crucial to most tasks required for cells to exist Catalysis – Enzymes speed up chemical reactions Defense – Antibodies and complement proteins attack pathogens Movement – Motor and contractile proteins move the cell or molecules within the cell © 2014 Pearson Education, Inc.
What Do Proteins Do? Signaling – Proteins convey signals between cells Structure – Structural proteins define cell shape and comprise body structures Transport – Transport proteins carry materials – Membrane proteins control molecular movement into and out of the cell © 2014 Pearson Education, Inc.
An Introduction to Catalysis may be the most fundamental of protein functions Reactions take place when – Reactants collide in precise orientation – Reactants have enough kinetic energy to overcome repulsion between the electrons that come in contact during bond formation Enzymes perform two functions: 1. Bring substrates together in precise orientation so that the electrons involved in the reaction can interact 2. Decrease the amount of kinetic energy that reactants must have for the reaction to proceed © 2014 Pearson Education, Inc.
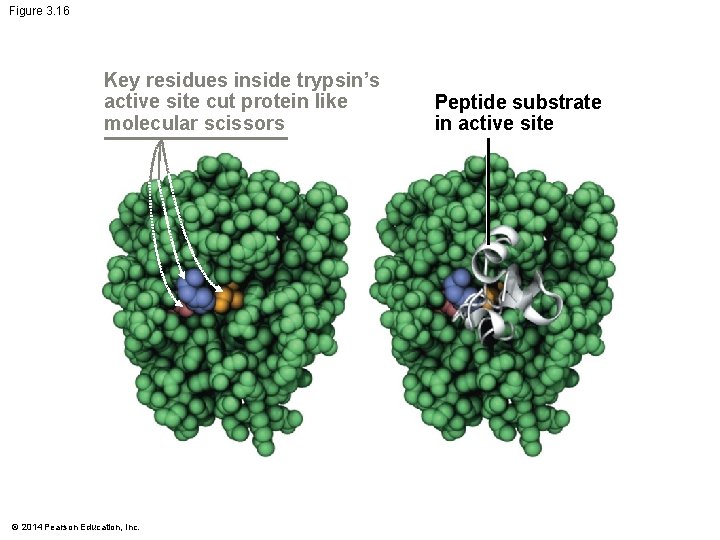
Figure 3. 16 Key residues inside trypsin’s active site cut protein like molecular scissors © 2014 Pearson Education, Inc. Peptide substrate in active site
- Slides: 52