Biological Oxygen Demand BOD Introduction Dissolved oxygen amount


Biological Oxygen Demand BOD
Introduction ü Dissolved oxygen – amount of actual oxygen dissolved in a water sample. Higher number = purer water ü BOD – Actual amount of dissolved oxygen metabolized over 5 days. Higher number = dirtier ü BOD – Amount of oxygen that would be needed to completely metabolize organic waste. Higher number = dirtier
BOD The biochemical oxygen demand is defined as the ‘measure of dissolve oxygen require to decompose the organic matter in water biologically. Pure water has < 1 ppm Polluted water >5 ppm
BOD Ø Normally, it is measured, Ø during 5 days Ø At 20 º Celsius Ø In the dark (to prevent algae growth)

BOD Ø “Biochemical Oxygen Demand” (BOD) was selected in 1908 as an indicator of the organic pollution of rivers by the U. K Ø This parameter was adopted by the American Public Health Association Standard Methods Committee in 1936 as a reference indicator to evaluate the biodegradation of chemicals and hazardous substances. Ø Five day period to estimate the BOD in river parameter was chosen for test.

BOD Ø Since most aquatic organisms need oxygen to carry out anaerobic respiration/ photosynthesis. Ø Water with High BOD can’t replenish oxygen fast enough, would not be able to meet the needs of the aquatic community. Ø Hence they will eventually suffocate
Standard Definition Of BOD This parameter is defined as “The amount of oxygen, divided by the volume of the system, taken up through the respiratory activity of microorganisms growing on the organic compounds present in the sample (e. g. water or sludge) when incubated at a specified temperature (usually 20 °C) for a fixed period (usually 5 days, BOD 5)”.
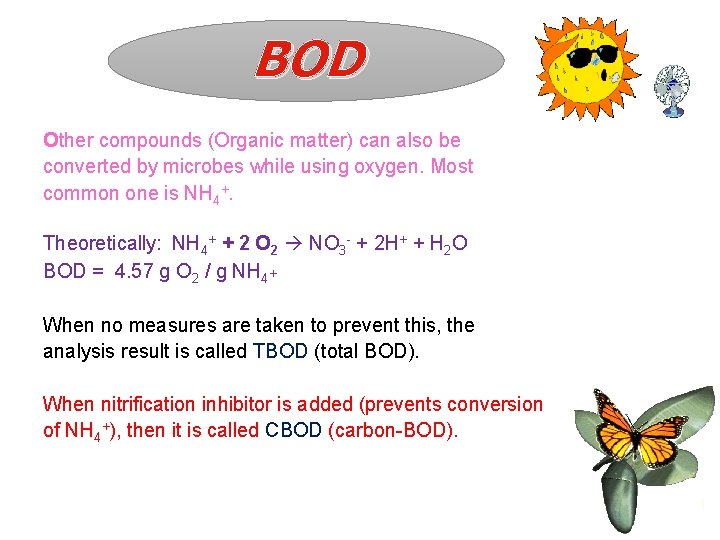
BOD Other compounds (Organic matter) can also be converted by microbes while using oxygen. Most common one is NH 4+. Theoretically: NH 4+ + 2 O 2 NO 3 - + 2 H+ + H 2 O BOD = 4. 57 g O 2 / g NH 4+ When no measures are taken to prevent this, the analysis result is called TBOD (total BOD). When nitrification inhibitor is added (prevents conversion of NH 4+), then it is called CBOD (carbon-BOD). 1
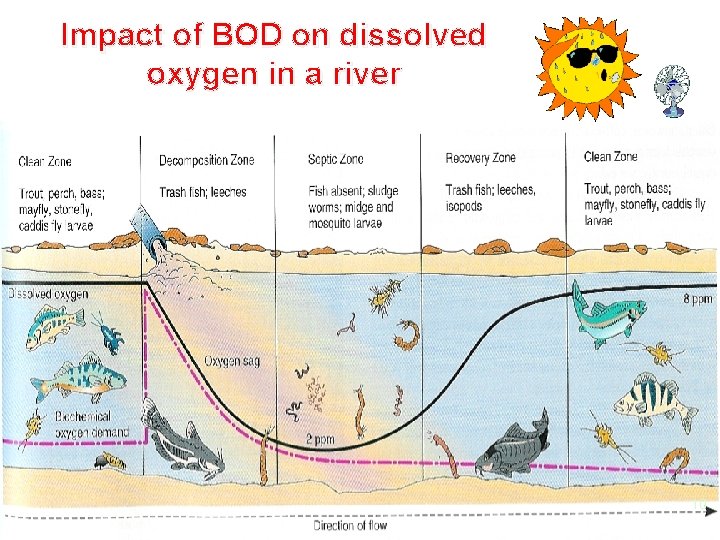
Impact of BOD on dissolved oxygen in a river 10
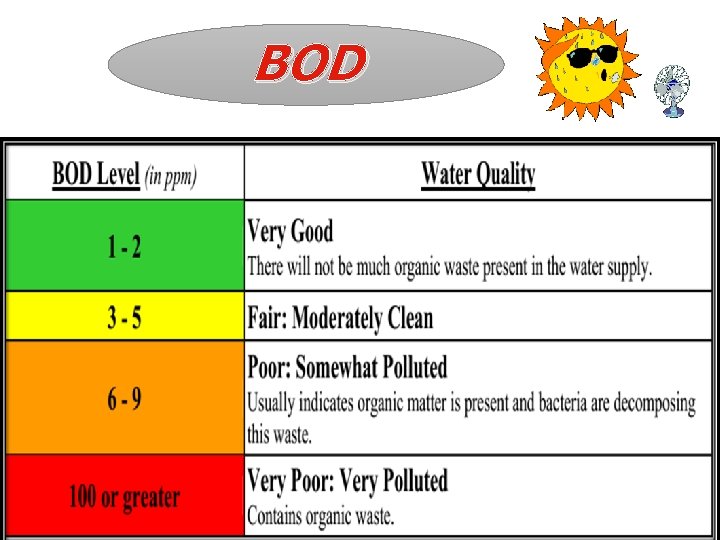
BOD

BOD ü Most natural waters contain small quantities of organic compounds. ü Microorganisms living in oxygenated waters use dissolved oxygen to degrade the organic compounds. ü Microbial metabolism can consume dissolved oxygen faster than atmospheric oxygen can dissolve into the water. ü Fish and aquatic insects may die when oxygen is depleted by microbial metabolism.
Methods of BOD measurement Standard nanometric method Dilution of samples with high organic loads, incubation at 20 °C in a dark room, in presence of microbial population for 5 days. ü The sample bottles are filled with a measured volume of sample. ü The microorganisms degrade organic substances using the gaseous oxygen trapped in the closed bottle. ü The carbon dioxide formed by this process is absorbed, generally with sodium hydroxide pellets. ü The pressure changes are measured by a manometer and converted to oxygen consumption by the device to estimate the BOD value.
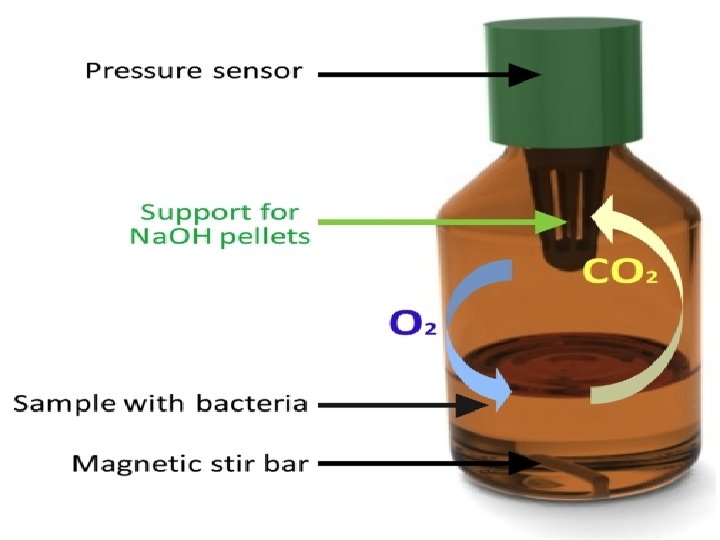
Applications BOD has three major applications 1 - It is used as an indicator of the conformity of the wastewater discharge and the waste treatment procedure to the current regulations. 2 - In wastewater treatment plants, the ratio between BOD 5 and COD (chemical oxygen demand) indicates the biodegradable fraction of an effluent. 3 - The ratio COD/BOD 5 is an indicator of the size of a wastewater treatment plant required for a specific location

CHEMICAL OXYGEN DEMAND
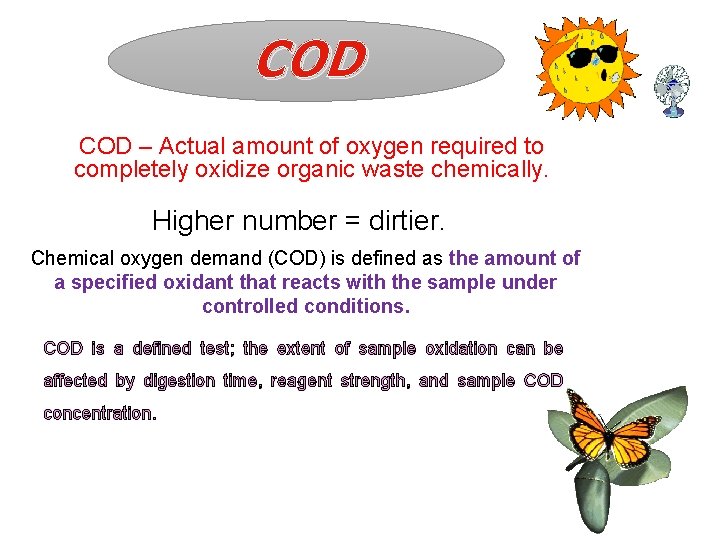
COD – Actual amount of oxygen required to completely oxidize organic waste chemically. Higher number = dirtier. Chemical oxygen demand (COD) is defined as the amount of a specified oxidant that reacts with the sample under controlled conditions. COD is a defined test; the extent of sample oxidation can be affected by digestion time, reagent strength, and sample COD concentration.
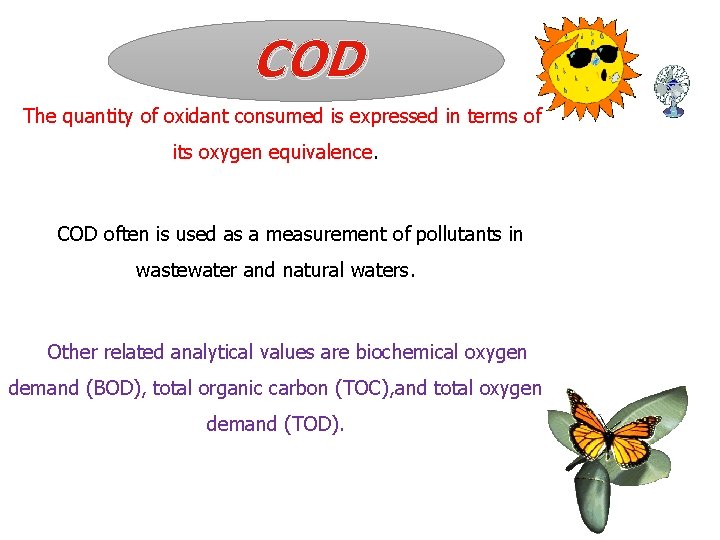
COD The quantity of oxidant consumed is expressed in terms of its oxygen equivalence. COD often is used as a measurement of pollutants in wastewater and natural waters. Other related analytical values are biochemical oxygen demand (BOD), total organic carbon (TOC), and total oxygen demand (TOD).
Terms BOD is a measure of oxygen consumed by microorganisms under specific conditions. TOC (total organic carbon ) is a measure of organic carbon in a sample. TOD (total oxygen demand (TOD). )is a measure of the amount of oxygen consumed by all elements in a sample when complete (total) oxidation is achieved.
COD Because of its unique chemical properties, dichromate ion (Cr 2 O 7 2 - )is the specified oxidant in various methods used for COD analysis; it is reduced to the chromic ion (Cr 3+) in these tests. Both organic and inorganic components of a sample are subject to oxidation, but in most cases the organic component predominates and is of the greater interest. In a COD analysis, hazardous wastes of mercury, hexavalent chromium, sulfuric acid, silver and acids are generated.
Methods for COD Analysis
Open Reflux Method Principle: Most types of organic matter are oxidized by a boiling mixture of chromic and sulfuric acids. After digestion, the remaining unreduced K 2 Cr 2 O 7 is titrated with ferrous ammonium sulfate to determine the amount of K 2 Cr 2 O 7 consumed and the oxidizable matter is calculated in terms of oxygen equivalent.
COD The most common COD errors are due to oxidation of inorganic species. Dichromate is a powerful oxidant – it will oxidize not only almost all organics but many metals and non-metal ions
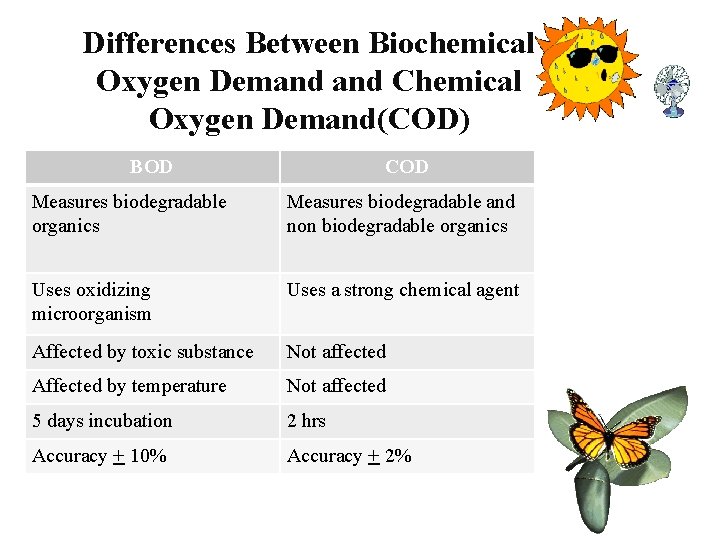
Differences Between Biochemical Oxygen Demand Chemical Oxygen Demand(COD) BOD COD Measures biodegradable organics Measures biodegradable and non biodegradable organics Uses oxidizing microorganism Uses a strong chemical agent Affected by toxic substance Not affected Affected by temperature Not affected 5 days incubation 2 hrs Accuracy + 10% Accuracy + 2%

Good Luck
- Slides: 25