BIOLOGICAL MOLECULES 3 TYPES CARBOHYDRATES LIPIDS PROTEINS Lipids
BIOLOGICAL MOLECULES 3 TYPES ! • CARBOHYDRATES • LIPIDS • PROTEINS
Lipids
What are lipids used for?

LIPIDS • “Lipid” is a name given to describe a range of substances • Some important lipids are a group called the triglycerides – usually known as fats and oils • Triglycerides are made up of three fatty acid molecules joined onto a molecule of glycerol.
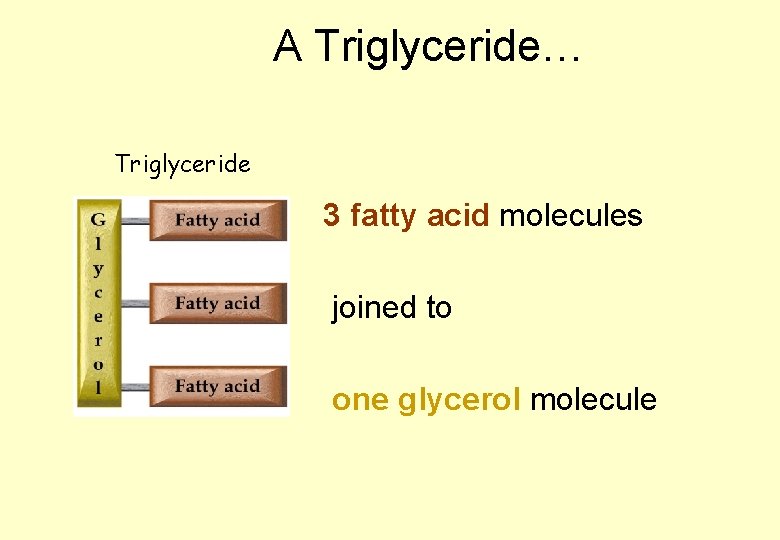
A Triglyceride… Triglyceride 3 fatty acid molecules joined to one glycerol molecule
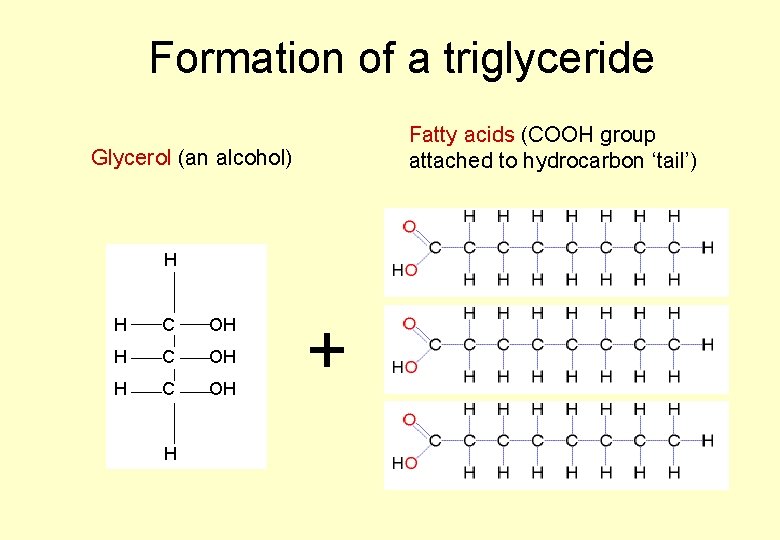
Formation of a triglyceride Fatty acids (COOH group attached to hydrocarbon ‘tail’) Glycerol (an alcohol) H H C OH H +
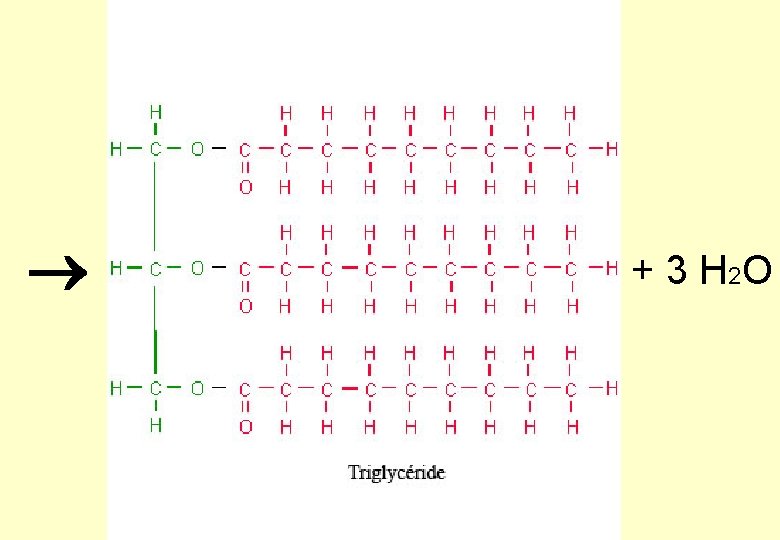
+ 3 H 2 O

Properties of lipids 1. They contain the elements C, H and O 2. They are insoluble in water 3. They can be saturated or unsaturated

2. Insolubility in water Lipids are insoluble in water – indeed, lipids’ functions are often related to their water repellent properties… * They are said to be HYDROPHOBIC water hating
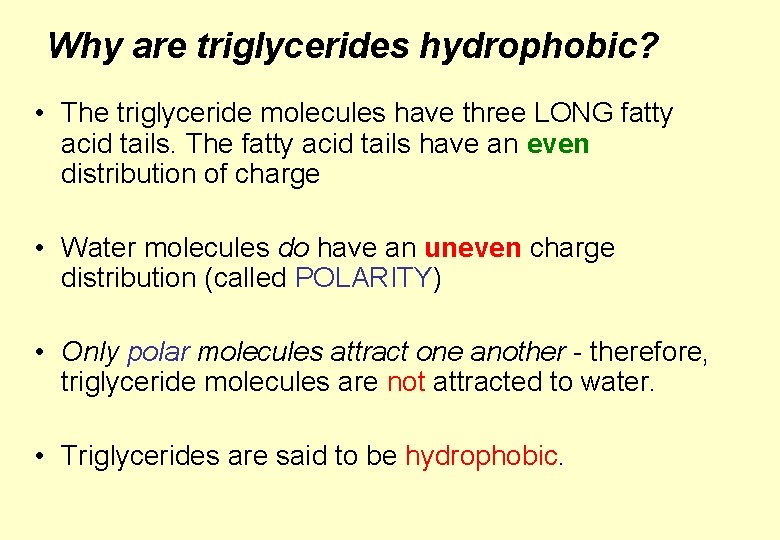
Why are triglycerides hydrophobic? • The triglyceride molecules have three LONG fatty acid tails. The fatty acid tails have an even distribution of charge • Water molecules do have an uneven charge distribution (called POLARITY) • Only polar molecules attract one another - therefore, triglyceride molecules are not attracted to water. • Triglycerides are said to be hydrophobic.
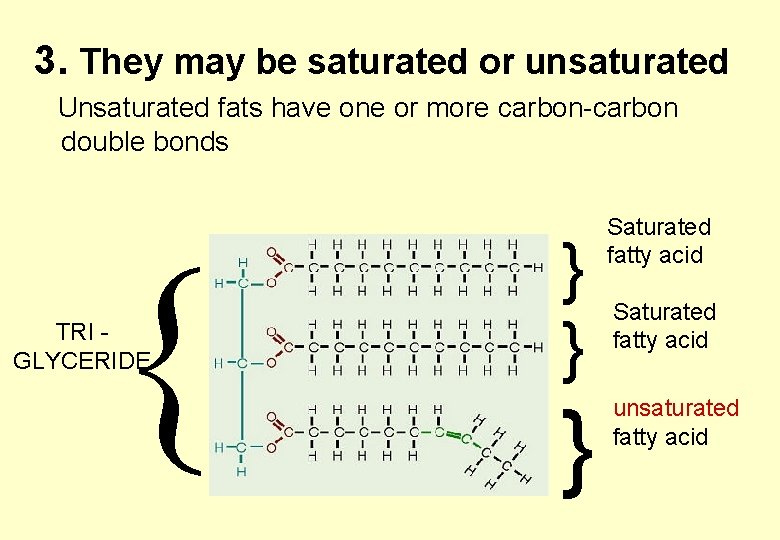
3. They may be saturated or unsaturated Unsaturated fats have one or more carbon-carbon double bonds TRI GLYCERIDE } } } Saturated fatty acid unsaturated fatty acid
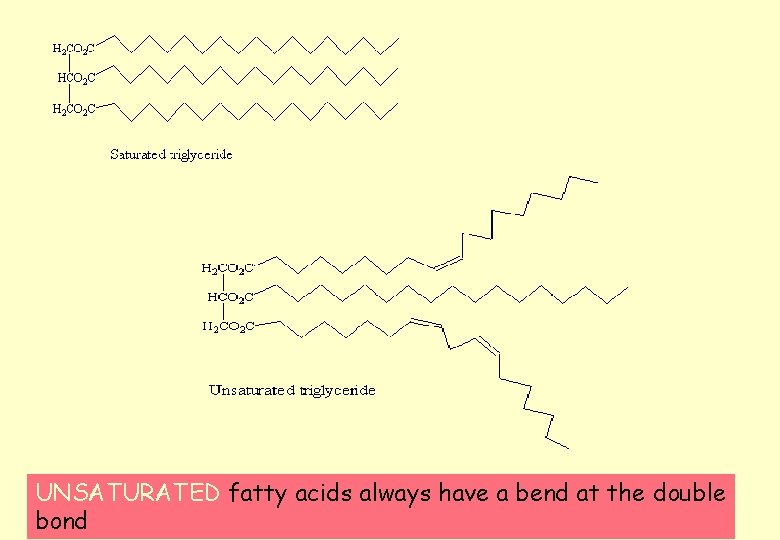
UNSATURATED fatty acids always have a bend at the double bond

Unsaturated fats • The presence of double bonds produces kinks in the hydrocarbon chains. • This stops the chains of neighbouring triglycerides lying too close together. • This makes the lipid more fluid therefore lowering its melting point. • Unsaturated fats are more likely to be liquids at room temperature – we refer to them as oils.

Mono- & Poly-unsaturates If there is more than one carbon-carbon double bond in the triglyceride molecule, the lipid is said to be POLYUNSATURATED
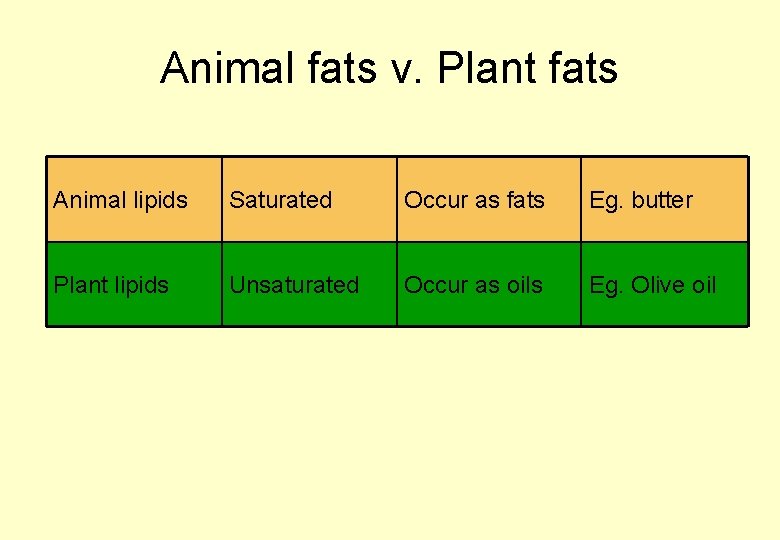
Animal fats v. Plant fats Animal lipids Saturated Occur as fats Eg. butter Plant lipids Unsaturated Occur as oils Eg. Olive oil

In addition to triglycerides, there are other types of lipids we must consider: • Waxes: Waterproofing: Feathers, leaf cuticles • Steroids: Small hydrophobic molecules in animals: cholesterol, oestrogen, cortisol • Terpenes: Small hydrophobic molecules in plants: vitamin A, menthol, plant oils • Phospholipids: Cell membranes…. cell…

Phospholipids are very similar to triglycerides EXCEPT One fatty acid group is replaced by a PHOSPHATE group which has an uneven electrical charge So how might they behave in water?
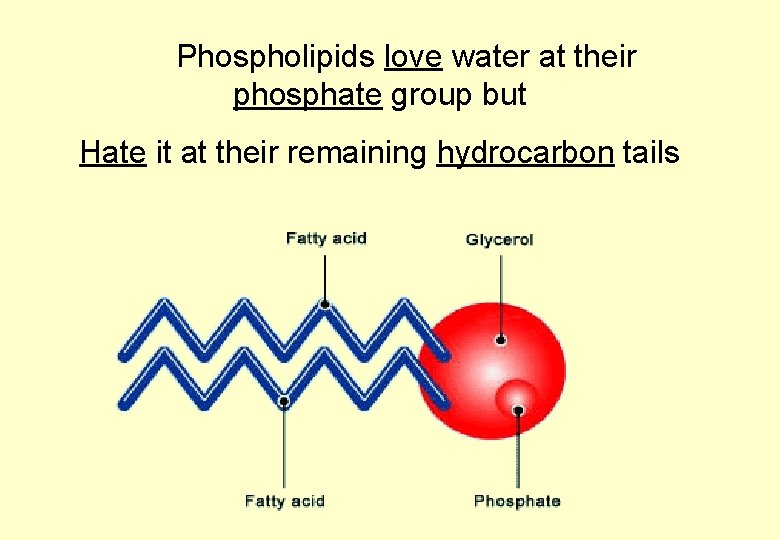
Phospholipids love water at their phosphate group but Hate it at their remaining hydrocarbon tails
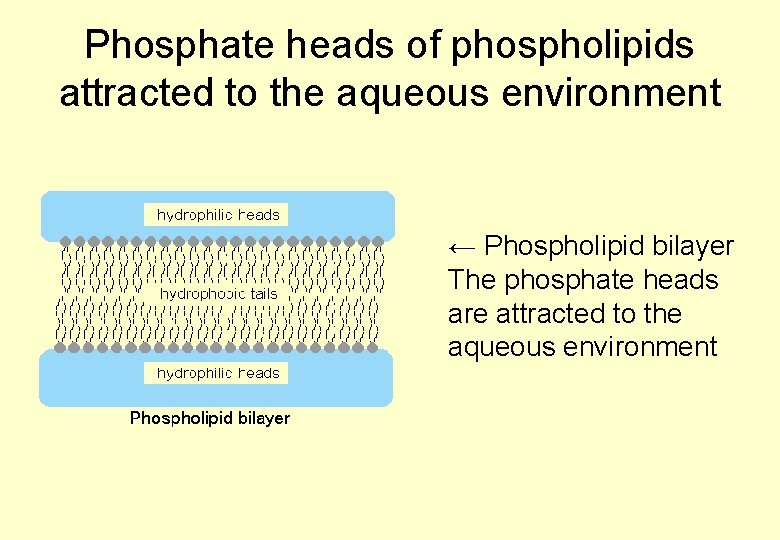
Phosphate heads of phospholipids attracted to the aqueous environment ← Phospholipid bilayer The phosphate heads are attracted to the aqueous environment
- Slides: 19