Biological Macromolecules Year 11 Biology Unit 1 Four

Biological Macromolecules Year 11 Biology Unit 1
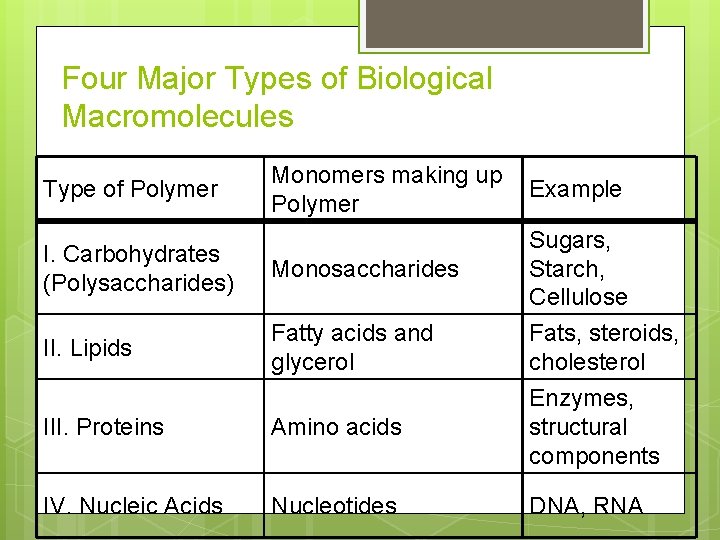
Four Major Types of Biological Macromolecules Monomers making up Polymer Example I. Carbohydrates (Polysaccharides) Monosaccharides Sugars, Starch, Cellulose II. Lipids Fatty acids and glycerol III. Proteins Amino acids IV. Nucleic Acids Nucleotides Type of Polymer Fats, steroids, cholesterol Enzymes, structural components DNA, RNA
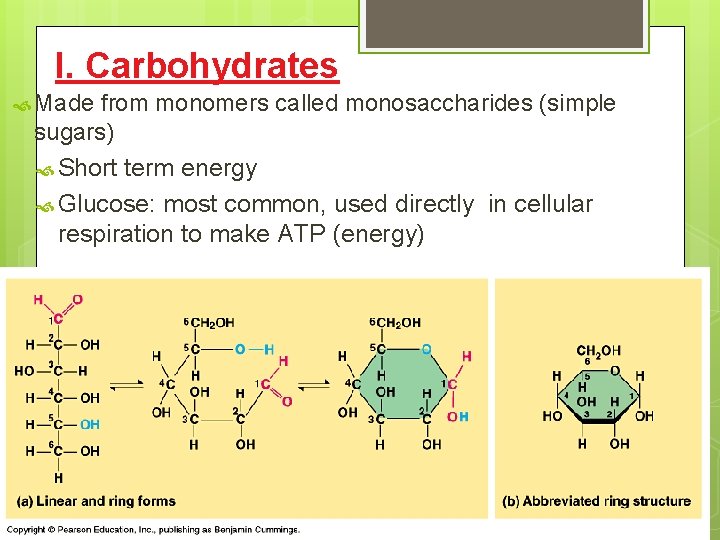
I. Carbohydrates Made from monomers called monosaccharides (simple sugars) Short term energy Glucose: most common, used directly in cellular respiration to make ATP (energy)
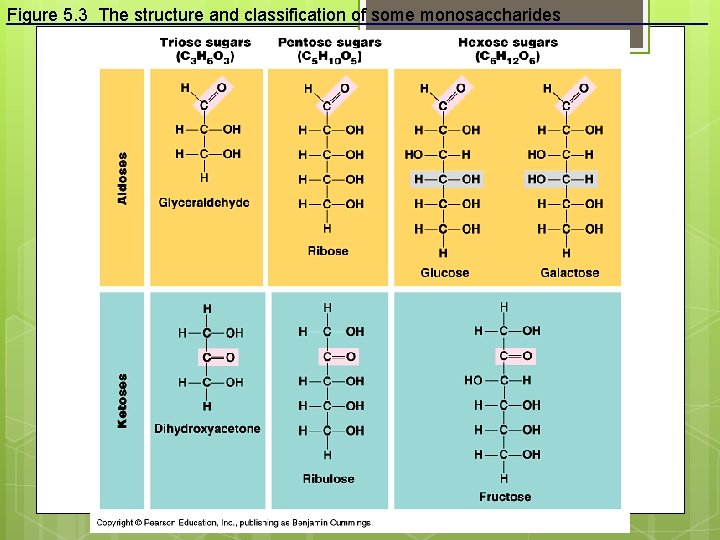
Figure 5. 3 The structure and classification of some monosaccharides
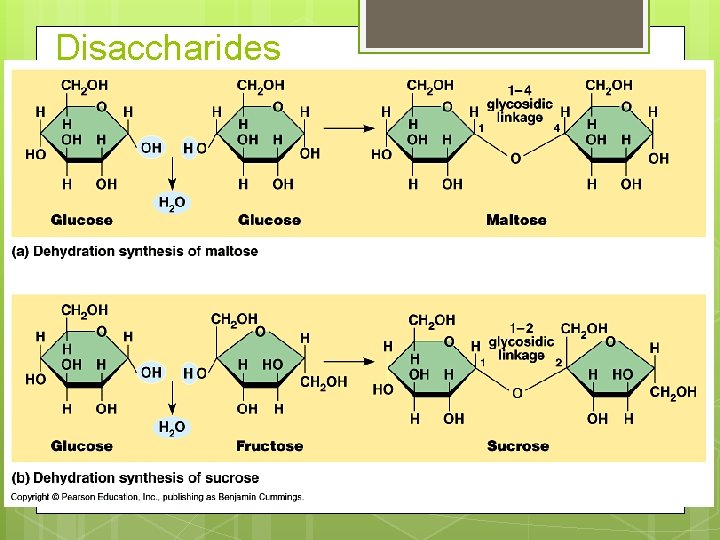
Disaccharides

Polysaccharides: Complex Carbohydrates 3 major types made from monomers of glucose: Starch: energy storage in plants Glycogen: energy storage in animals Cellulose: structural molecules in plants
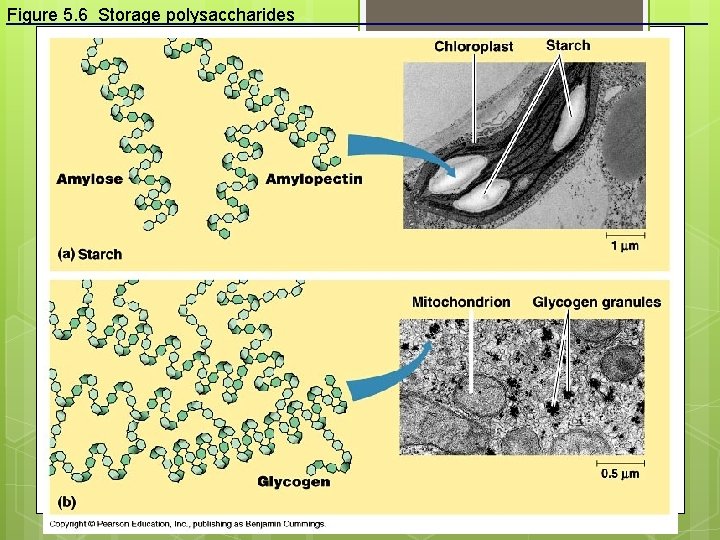
Figure 5. 6 Storage polysaccharides
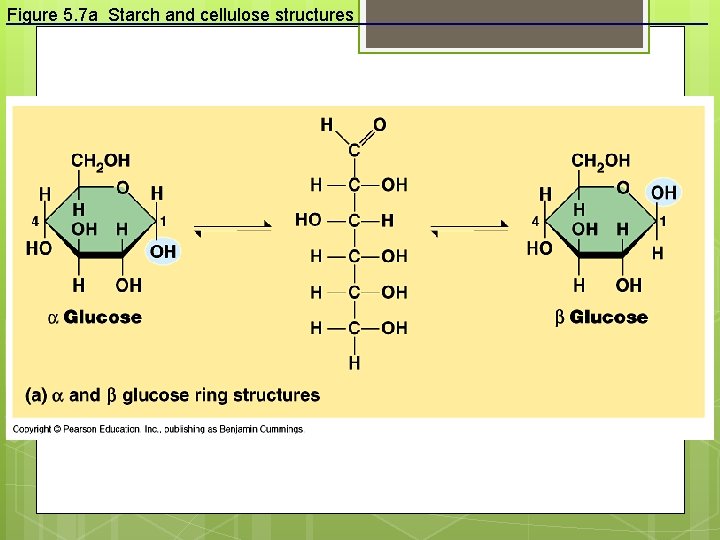
Figure 5. 7 a Starch and cellulose structures

Figure 5. 7 b, c Starch and cellulose structures
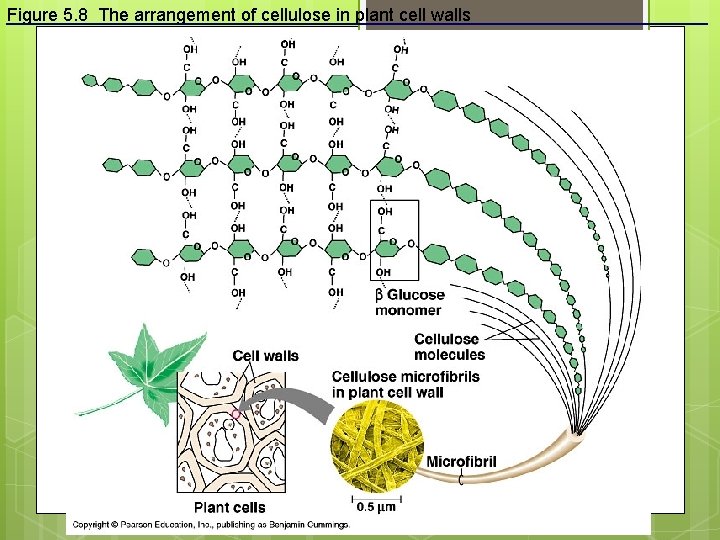
Figure 5. 8 The arrangement of cellulose in plant cell walls

II. Lipids Not true polymers Composed of mostly glycerol and fatty acids Includes Fats: energy storage Phospholipids: membranes Steroids: hormones, cholesterol
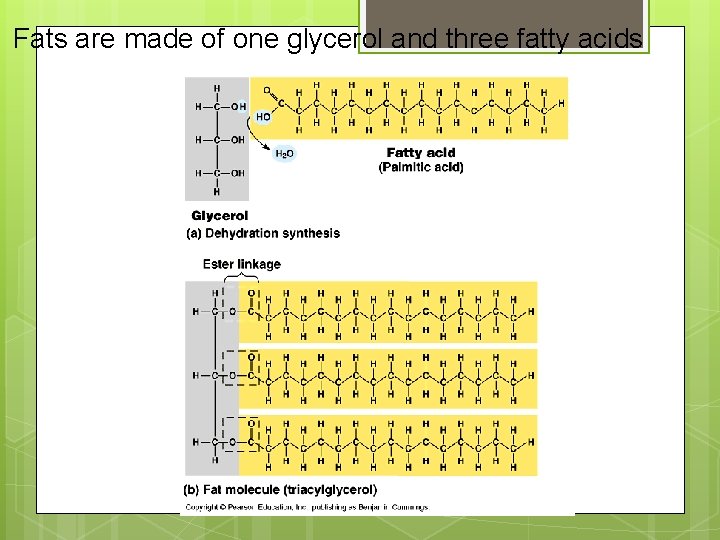
Fats are made of one glycerol and three fatty acids

Double bonds between carbons cause kinks in hydrocarbons. H 2 C CH 2 H 2 C CH Kink CH H 2 C CH 2 Unsaturated fatty acid Double bonds, fewer H atoms Saturated fatty acid No Double bonds, maximum H atoms
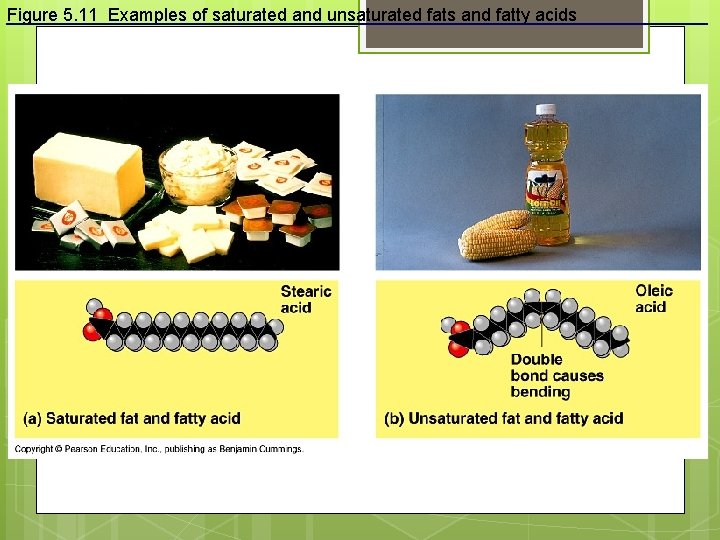
Figure 5. 11 Examples of saturated and unsaturated fats and fatty acids
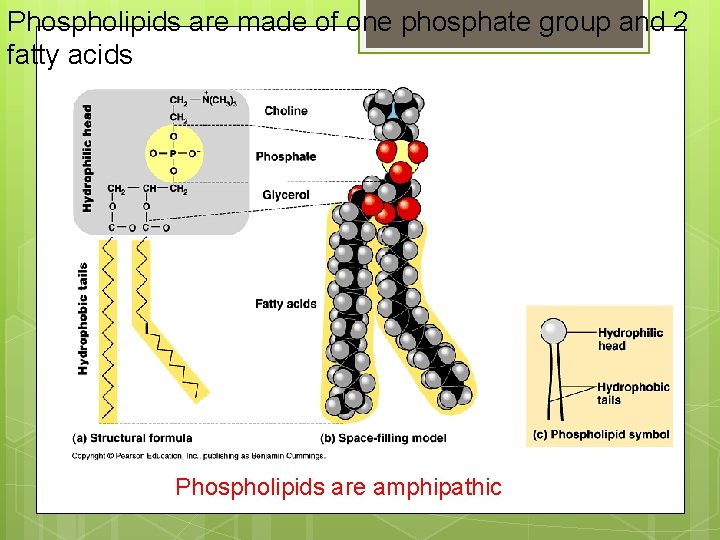
Phospholipids are made of one phosphate group and 2 fatty acids Phospholipids are amphipathic
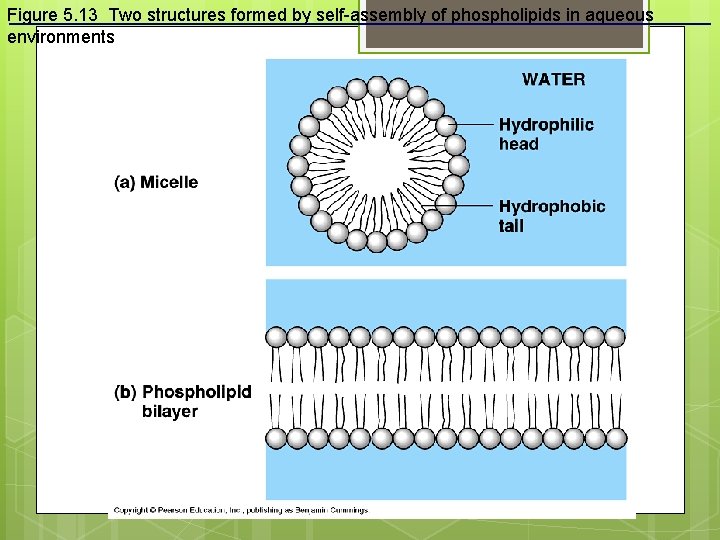
Figure 5. 13 Two structures formed by self-assembly of phospholipids in aqueous environments
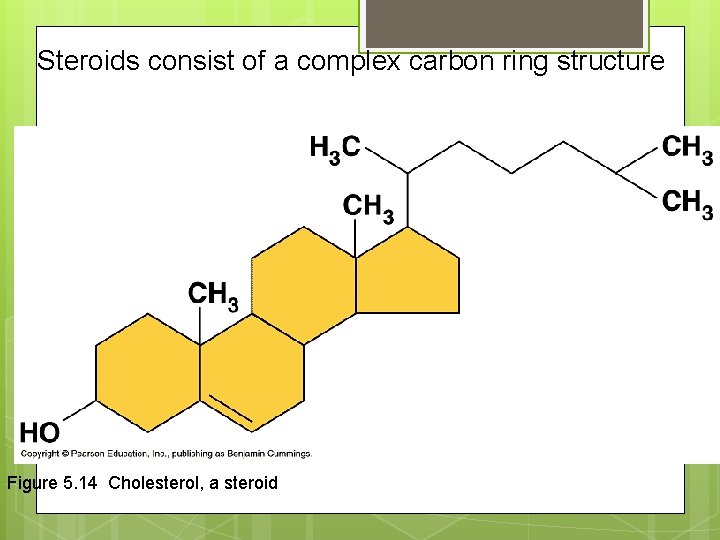
Steroids consist of a complex carbon ring structure Figure 5. 14 Cholesterol, a steroid
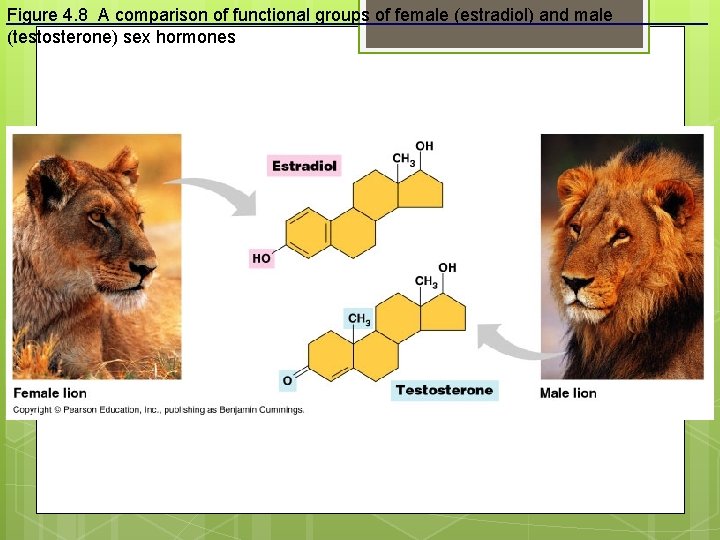
Figure 4. 8 A comparison of functional groups of female (estradiol) and male (testosterone) sex hormones
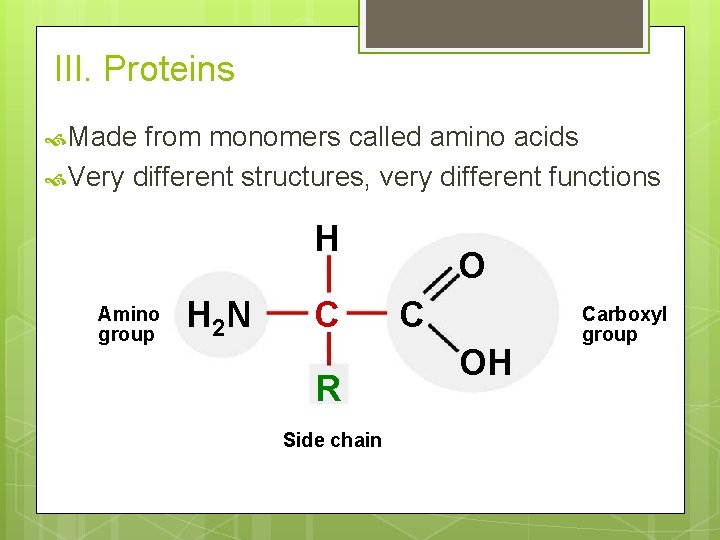
III. Proteins Made from monomers called amino acids Very different structures, very different functions H Amino group H 2 N C R Side chain O C OH Carboxyl group
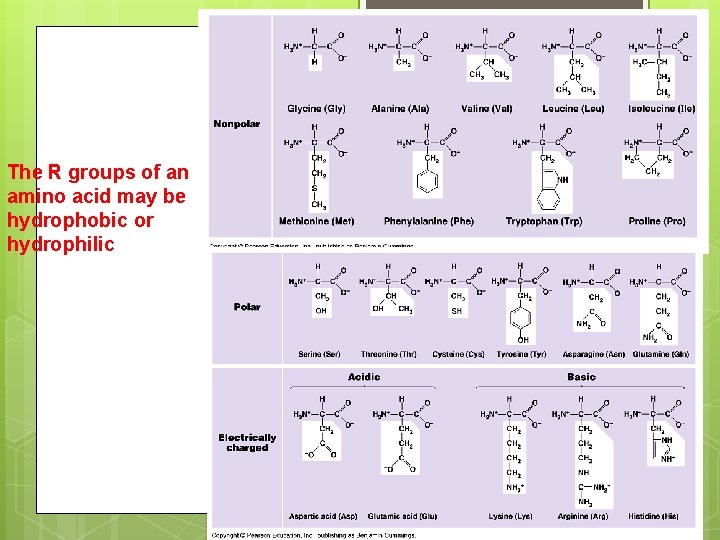
The R groups of an amino acid may be hydrophobic or hydrophilic
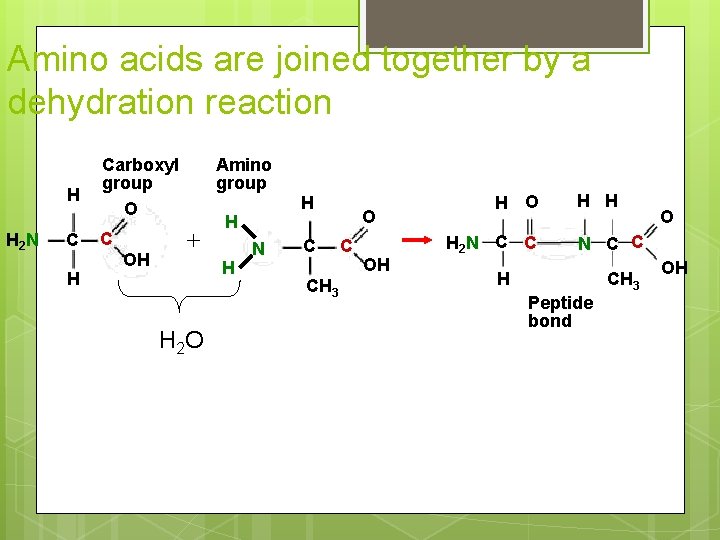
Amino acids are joined together by a dehydration reaction H H 2 N C H Carboxyl group O C OH Amino group + H H H 2 O H N C CH 3 O C OH H O H 2 N C C H H O N C C H Peptide bond CH 3 OH
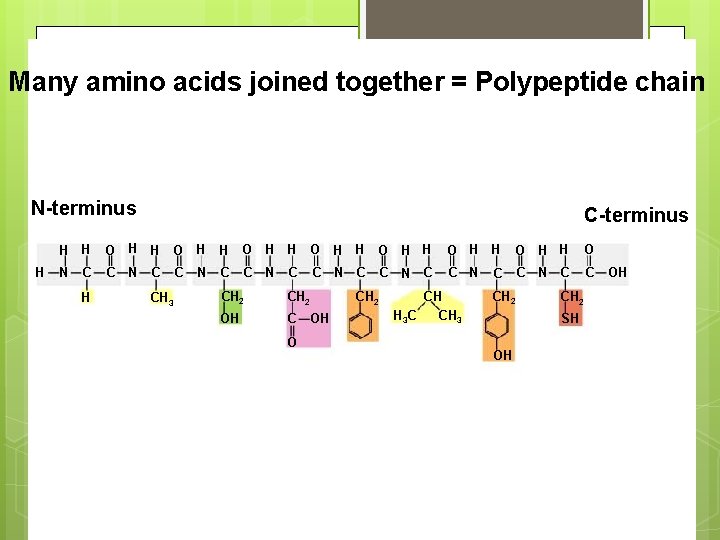
Many amino acids joined together = Polypeptide chain N-terminus H C-terminus H H O H H O N C C C C N C C C H C CH 3 N N C CH 2 OH C OH O CH 2 CH H 3 C CH 3 C CH 2 SH OH OH
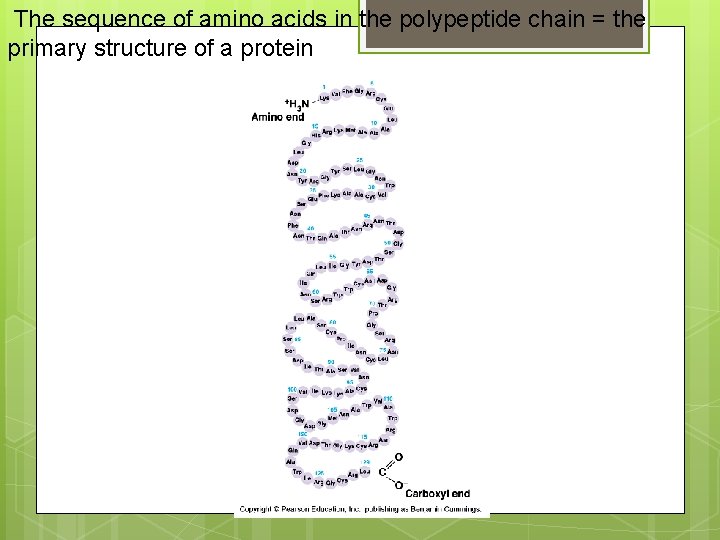
The sequence of amino acids in the polypeptide chain = the primary structure of a protein
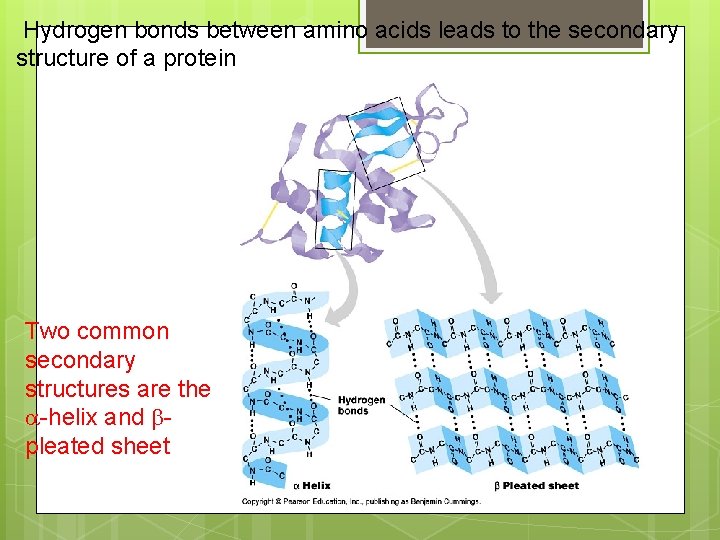
Hydrogen bonds between amino acids leads to the secondary structure of a protein Two common secondary structures are the -helix and pleated sheet
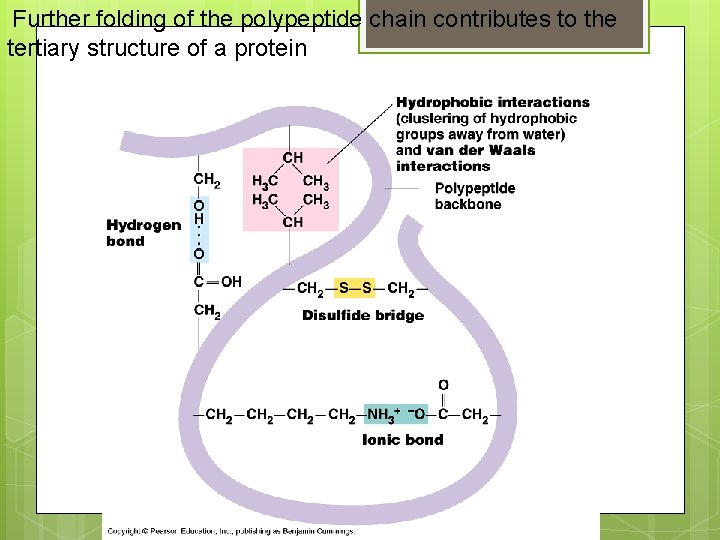
Further folding of the polypeptide chain contributes to the tertiary structure of a protein

The joining of more than one polypeptide chain leads to the quaternary structure of proteins
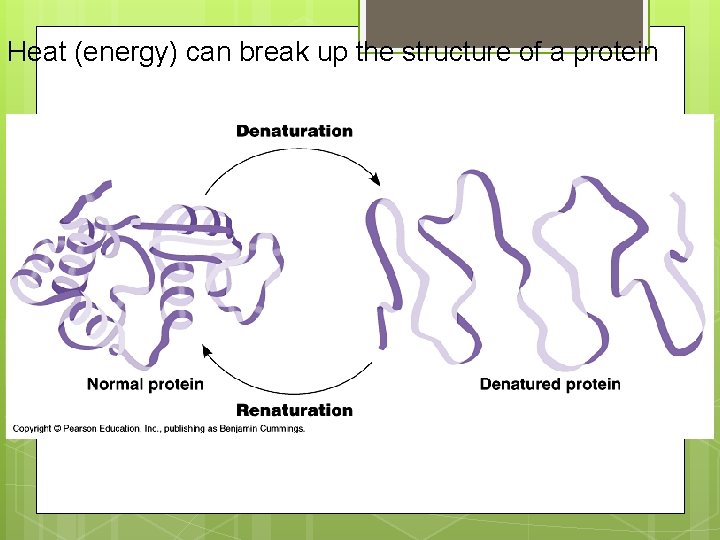
Heat (energy) can break up the structure of a protein
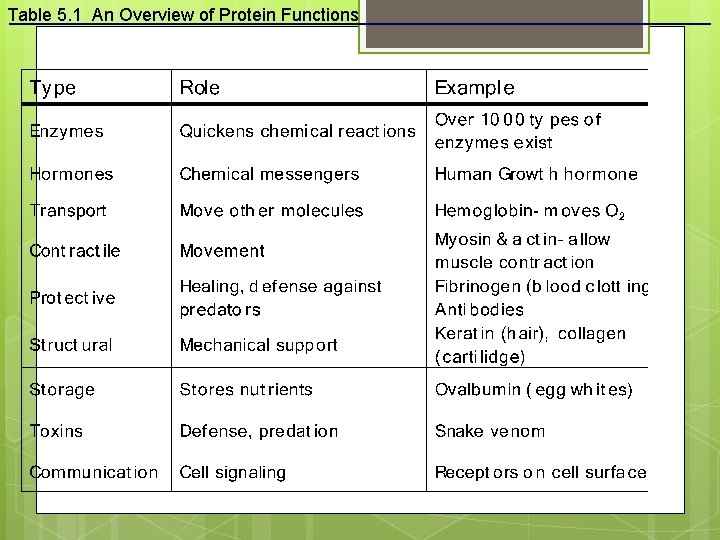
Table 5. 1 An Overview of Protein Functions
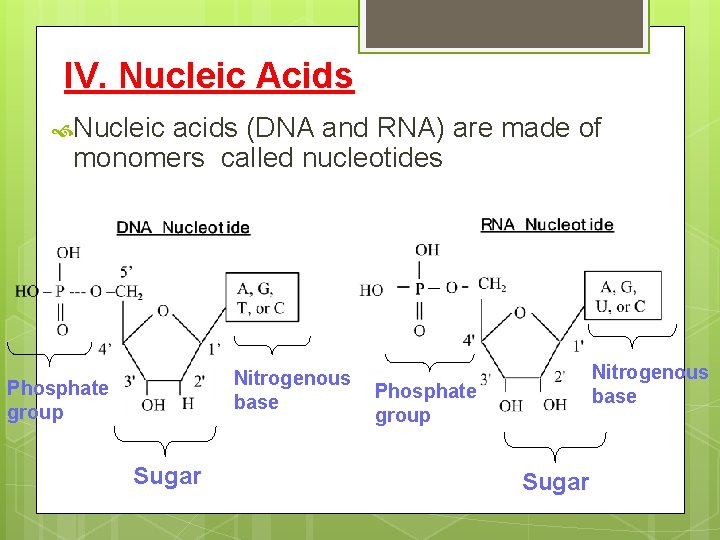
IV. Nucleic Acids Nucleic acids (DNA and RNA) are made of monomers called nucleotides Nitrogenous base Phosphate group Sugar
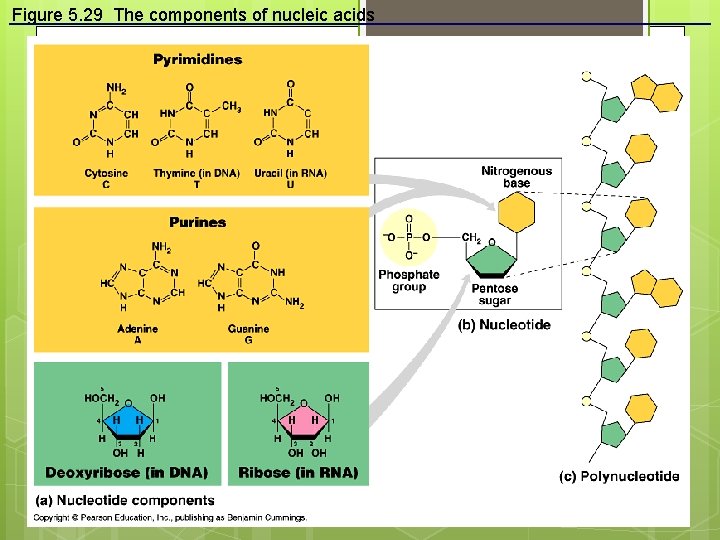
Figure 5. 29 The components of nucleic acids
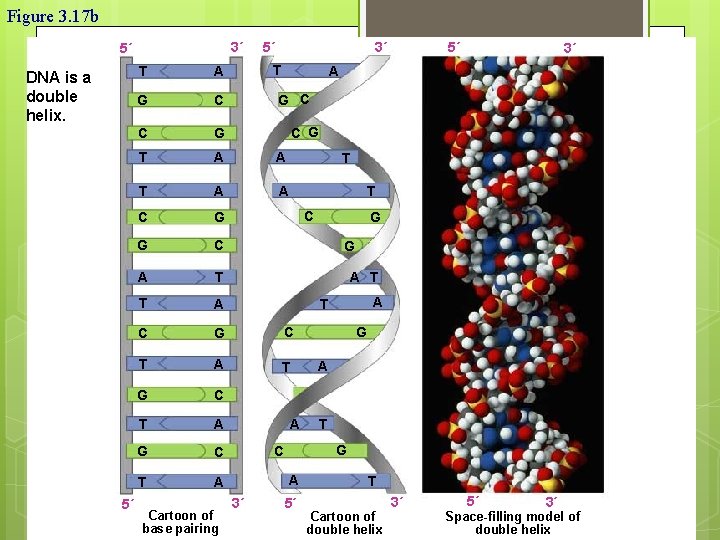
Figure 3. 17 b 3´ 5´ DNA is a double helix. 5´ 5´ 3´ T T A G C C G T A A C G G C A T T A C G C T A T G C T A Cartoon of base pairing 5´ 3´ A G C C G T T C G G A T A A T G C A 3´ G 5´ T Cartoon of double helix 3´ 5´ 3´ Space-filling model of double helix

Nucleic acids store the information to make proteins
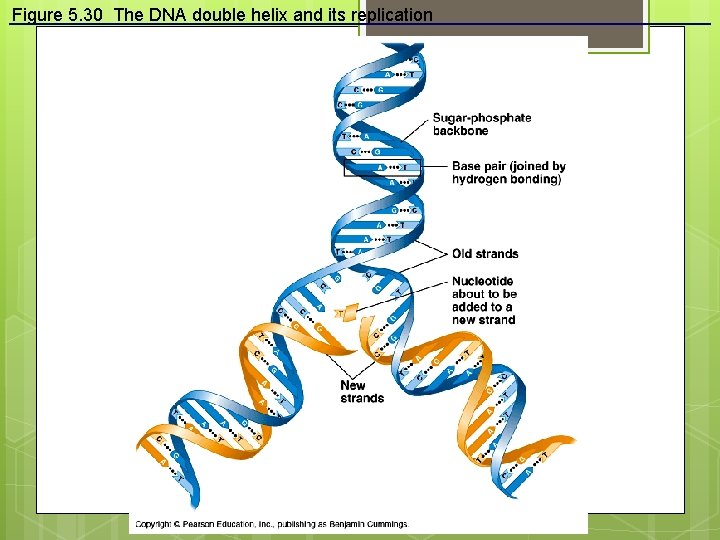
Figure 5. 30 The DNA double helix and its replication
- Slides: 33