BIOL 200 Section 921 Lecture 11 July 4
![BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton • BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton •](https://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-1.jpg)
BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton • Reading: • ECB, 2 nd ed. Chap 17. pp 573 -606; Questions 17 -1, 17 -2, 17 -12 to 17 -23. • ECB, 1 st ed. Chap 16. pp 513 -542; Questions 16 -1, 16 -2, 16 -10 to 16 -21.
UNIT 8: Cytoskeleton - Objectives 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. Two major roles for cytoskeleton - skeletal support and motility Distinguish between three major cytoskeletal systems - intermediate filaments, microtubules and actin filaments (microfilaments). Be able to describe how intermediate filaments are assembled from polypeptides to form a microscopically visible fibre. Know major cell function of intermediate filaments Know structure of microtubules, their process of assembly, the meaning of plus and minus ends, and the role of MTOCs. Understand dynamic instability and how it may be applied to microtubules and microtubule containing structures. Understand the role of GTP in the generation and control of dynamic instability of microtubules. Understand how motor proteins work and how their movement relates to the polarity of their molecular substrates Be able to describe the structure of flagella and the molecular basis of flagellar bending assembly of actin filaments, dynamic instability of actin filaments; comparison with that of microtubules. role of actin filaments in formation of the cell cortex, and regulation of cell structure and movement, myosins and the myosin activity cycle as it relates to muscle.
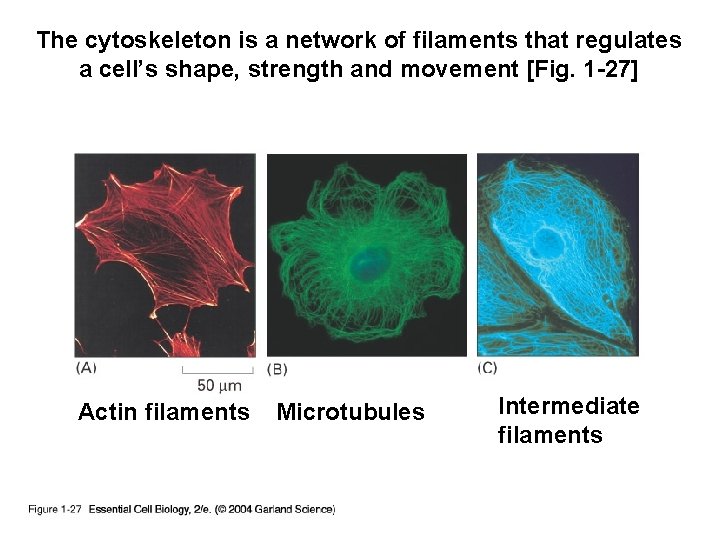
The cytoskeleton is a network of filaments that regulates a cell’s shape, strength and movement [Fig. 1 -27] Actin filaments Microtubules Intermediate filaments
![An overview of the cytoskeleton [Fig. 17 -2] Intermediate Filamentstough ropes Microtubulesbig hollow tubes An overview of the cytoskeleton [Fig. 17 -2] Intermediate Filamentstough ropes Microtubulesbig hollow tubes](http://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-4.jpg)
An overview of the cytoskeleton [Fig. 17 -2] Intermediate Filamentstough ropes Microtubulesbig hollow tubes support cell structures Actin Microfilamentshelical polymers involved in movement/shape

17_02_01_protein_filament. jpg
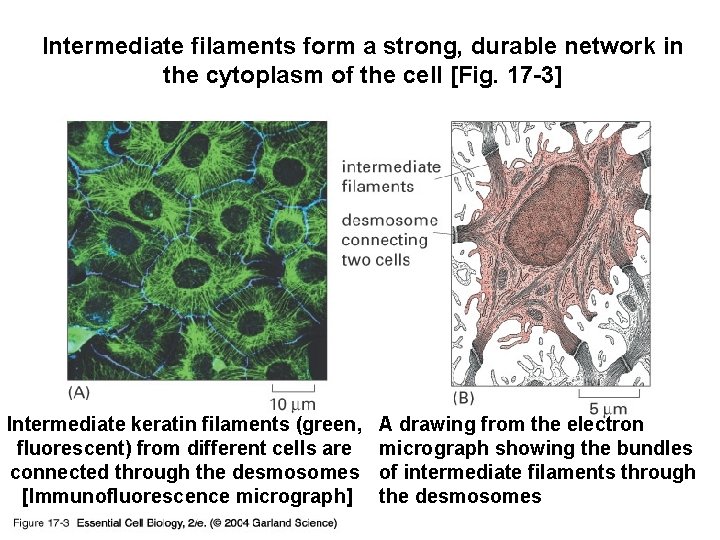
Intermediate filaments form a strong, durable network in the cytoplasm of the cell [Fig. 17 -3] 17_03_Interm_filaments. jpg Intermediate keratin filaments (green, fluorescent) from different cells are connected through the desmosomes [Immunofluorescence micrograph] A drawing from the electron micrograph showing the bundles of intermediate filaments through the desmosomes
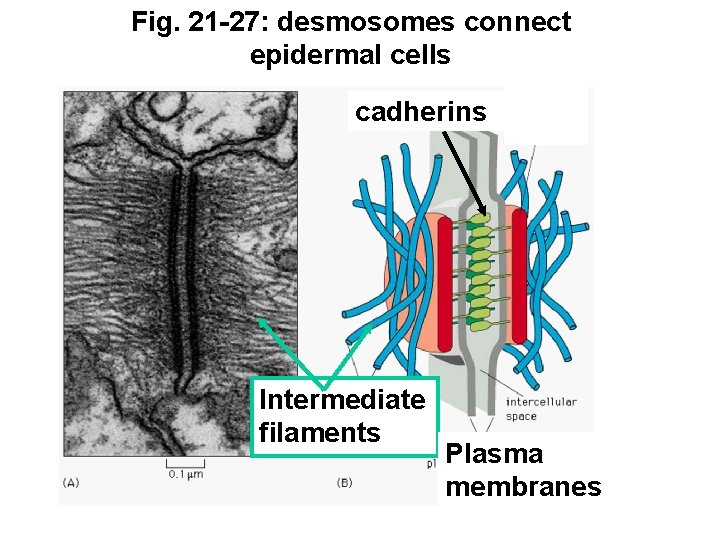
Fig. 21 -27: desmosomes connect epidermal cells cadherins Intermediate filaments Plasma membranes
![monomer dimer Assembly of intermediate filaments involves coiled coil dimers [Fig. 17 -4] monomer dimer Assembly of intermediate filaments involves coiled coil dimers [Fig. 17 -4]](http://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-8.jpg)
monomer dimer Assembly of intermediate filaments involves coiled coil dimers [Fig. 17 -4]
![Intermediate filaments strengthen animal cells [Fig. 17 -5] 17_05_strengthen_cells. jpg Intermediate filaments strengthen animal cells [Fig. 17 -5] 17_05_strengthen_cells. jpg](http://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-9.jpg)
Intermediate filaments strengthen animal cells [Fig. 17 -5] 17_05_strengthen_cells. jpg
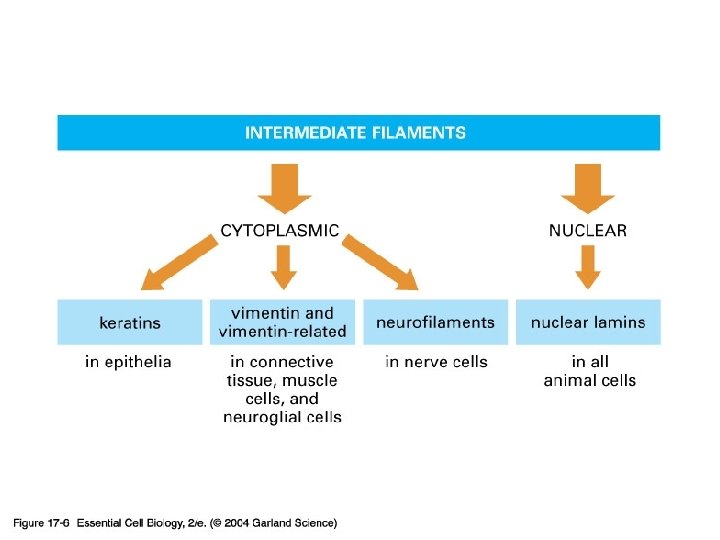
17_06_filam_categories. jpg
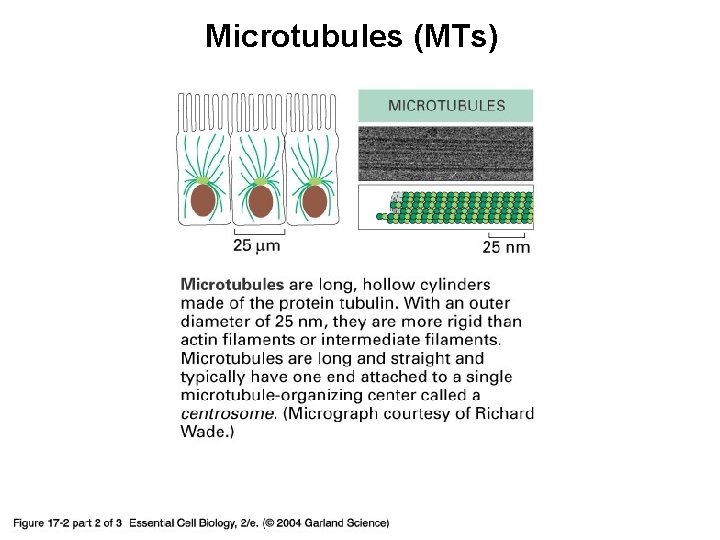
Microtubules (MTs) 17_02_02_protein_filament. jpg
![MTs grow from MT organizing centers [Fig. 17 -9] cilia centrosome Basal bodies Spindle MTs grow from MT organizing centers [Fig. 17 -9] cilia centrosome Basal bodies Spindle](http://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-12.jpg)
MTs grow from MT organizing centers [Fig. 17 -9] cilia centrosome Basal bodies Spindle poles
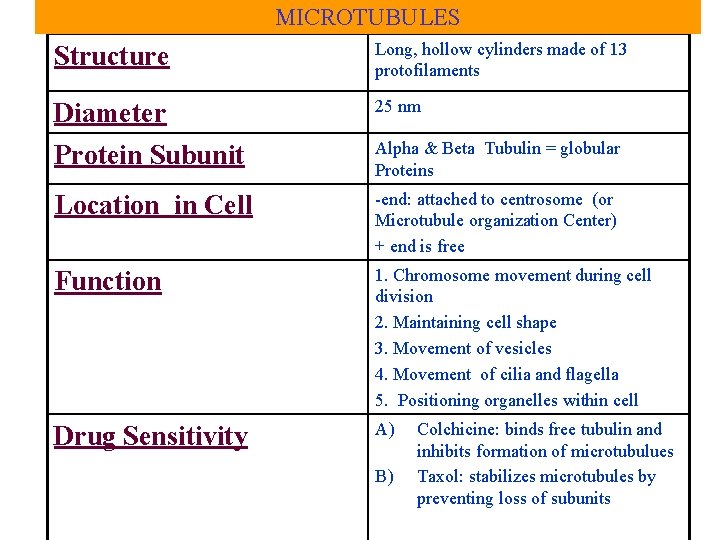
MICROTUBULES Structure Long, hollow cylinders made of 13 protofilaments Diameter Protein Subunit 25 nm Location in Cell -end: attached to centrosome (or Microtubule organization Center) + end is free Function 1. Chromosome movement during cell division 2. Maintaining cell shape 3. Movement of vesicles 4. Movement of cilia and flagella 5. Positioning organelles within cell Drug Sensitivity A) Alpha & Beta Tubulin = globular Proteins B) Colchicine: binds free tubulin and inhibits formation of microtubulues Taxol: stabilizes microtubules by preventing loss of subunits
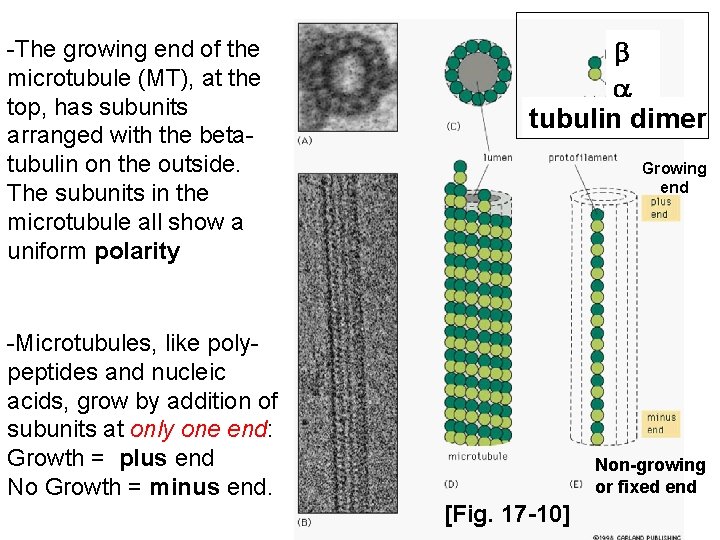
-The growing end of the microtubule (MT), at the top, has subunits arranged with the betatubulin on the outside. The subunits in the microtubule all show a uniform polarity b a tubulin dimer Growing end -Microtubules, like polypeptides and nucleic acids, grow by addition of subunits at only one end: Growth = plus end No Growth = minus end. Non-growing or fixed end [Fig. 17 -10]
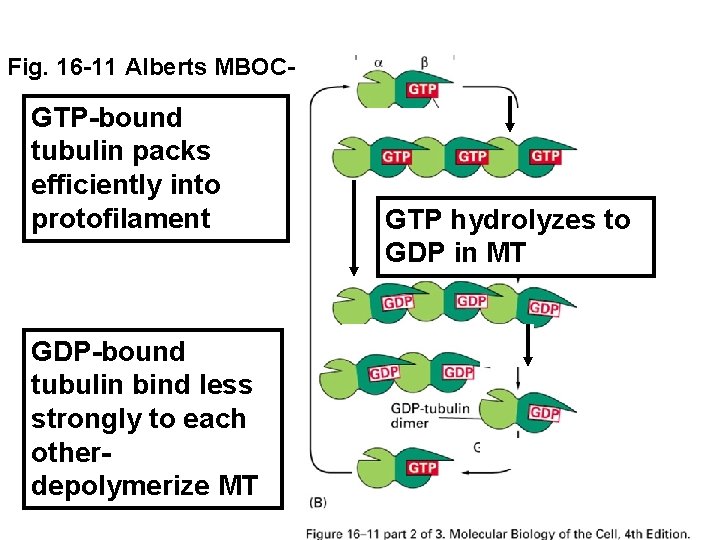
Fig. 16 -11 Alberts MBOC- GTP-bound tubulin packs efficiently into protofilament GDP-bound tubulin bind less strongly to each otherdepolymerize MT GTP hydrolyzes to GDP in MT
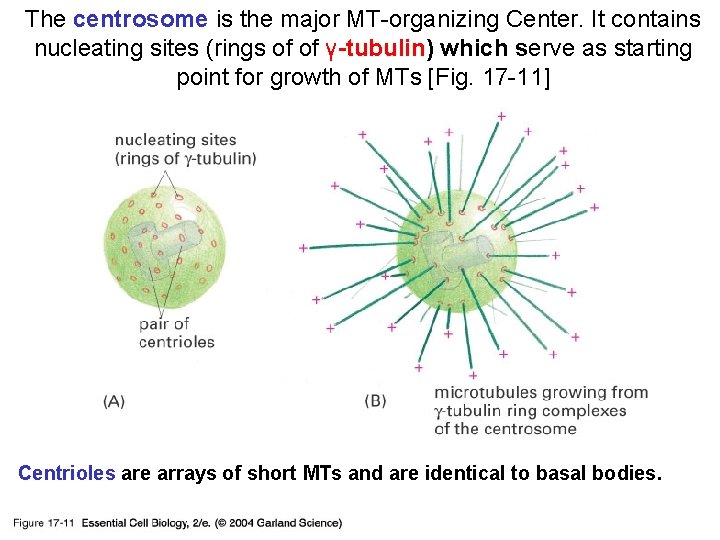
The centrosome is the major MT-organizing Center. It contains nucleating sites (rings of of γ-tubulin) which serve as starting point for growth of MTs [Fig. 17 -11] 17_11_centrosome. jpg Centrioles are arrays of short MTs and are identical to basal bodies.
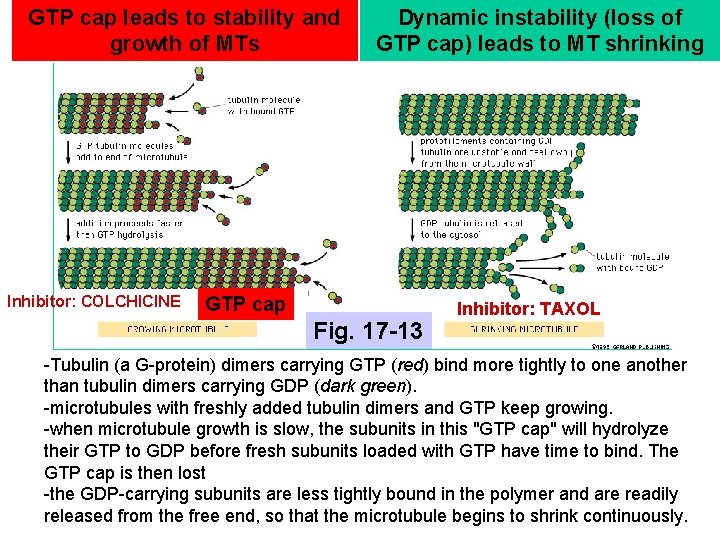
GTP cap leads to stability and growth of MTs Inhibitor: COLCHICINE Dynamic instability (loss of GTP cap) leads to MT shrinking GTP cap Fig. 17 -13 Inhibitor: TAXOL -Tubulin (a G-protein) dimers carrying GTP (red) bind more tightly to one another than tubulin dimers carrying GDP (dark green). -microtubules with freshly added tubulin dimers and GTP keep growing. -when microtubule growth is slow, the subunits in this "GTP cap" will hydrolyze their GTP to GDP before fresh subunits loaded with GTP have time to bind. The GTP cap is then lost -the GDP-carrying subunits are less tightly bound in the polymer and are readily released from the free end, so that the microtubule begins to shrink continuously.
![Three classes of MTs make up the mitotic spindle at metaphase [Fig. 19 -13] Three classes of MTs make up the mitotic spindle at metaphase [Fig. 19 -13]](http://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-18.jpg)
Three classes of MTs make up the mitotic spindle at metaphase [Fig. 19 -13] Aster MTs Kinetochore MTs Interpolar MTs
![Sister chromatids separate at anaphase [Fig. 19 -17] I. II. Sister chromatids separate at anaphase [Fig. 19 -17] I. II.](http://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-19.jpg)
Sister chromatids separate at anaphase [Fig. 19 -17] I. II.
![Each microtubule filament grows and shrinks independent of its neighbors [Fig. 17 -12] 17_12_grows_shrinks. Each microtubule filament grows and shrinks independent of its neighbors [Fig. 17 -12] 17_12_grows_shrinks.](http://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-20.jpg)
Each microtubule filament grows and shrinks independent of its neighbors [Fig. 17 -12] 17_12_grows_shrinks. jpg
![A model of microtubule assembly [Becker et al. The World of the Cell] A model of microtubule assembly [Becker et al. The World of the Cell]](http://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-21.jpg)
A model of microtubule assembly [Becker et al. The World of the Cell]
![The selective stabilization of MTs can polarize a cell [Fig. 17 -14] 17_14_polarize_cell. jpg The selective stabilization of MTs can polarize a cell [Fig. 17 -14] 17_14_polarize_cell. jpg](http://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-22.jpg)
The selective stabilization of MTs can polarize a cell [Fig. 17 -14] 17_14_polarize_cell. jpg • A MT can be stabilized by attaching its plus end to a capping protein or cell structure that prevents tubulin depolymerization • This is how organelles are positioned in cells
![Motor proteins [Dynein and Kinesin] transport vesicles along MTs in a nerve cell [Fig. Motor proteins [Dynein and Kinesin] transport vesicles along MTs in a nerve cell [Fig.](http://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-23.jpg)
Motor proteins [Dynein and Kinesin] transport vesicles along MTs in a nerve cell [Fig. 17 -15] cell body MT axon terminal - + dynein kinesin Nerve cell polarity maintained by microtubules
![Motor proteins [Dynein and Kinesin] move along MTs using their globular heads [Fig. 17 Motor proteins [Dynein and Kinesin] move along MTs using their globular heads [Fig. 17](http://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-24.jpg)
Motor proteins [Dynein and Kinesin] move along MTs using their globular heads [Fig. 17 -17] dynein kinesin
![Motor proteins transport their cargo along MTs [Fig. 17 -18] 17_18_motor_proteins. jpg Motor proteins transport their cargo along MTs [Fig. 17 -18] 17_18_motor_proteins. jpg](http://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-25.jpg)
Motor proteins transport their cargo along MTs [Fig. 17 -18] 17_18_motor_proteins. jpg
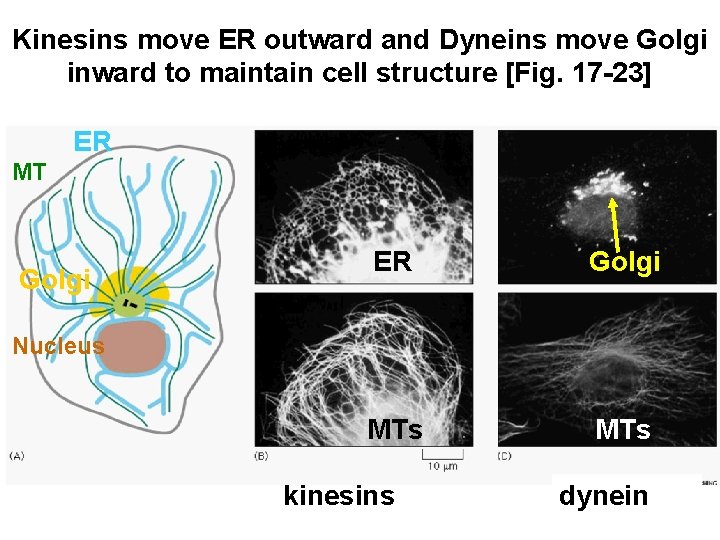
Kinesins move ER outward and Dyneins move Golgi inward to maintain cell structure [Fig. 17 -23] ER MT Golgi ER Golgi MTs Nucleus kinesins dynein
![Kinesin walks along a MT [Fig. 17 -22] 17_22_kinesin_moves. jpg Heads Kinesin-GFP moves along Kinesin walks along a MT [Fig. 17 -22] 17_22_kinesin_moves. jpg Heads Kinesin-GFP moves along](http://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-27.jpg)
Kinesin walks along a MT [Fig. 17 -22] 17_22_kinesin_moves. jpg Heads Kinesin-GFP moves along a MT Moves in a Series of 8 nm steps
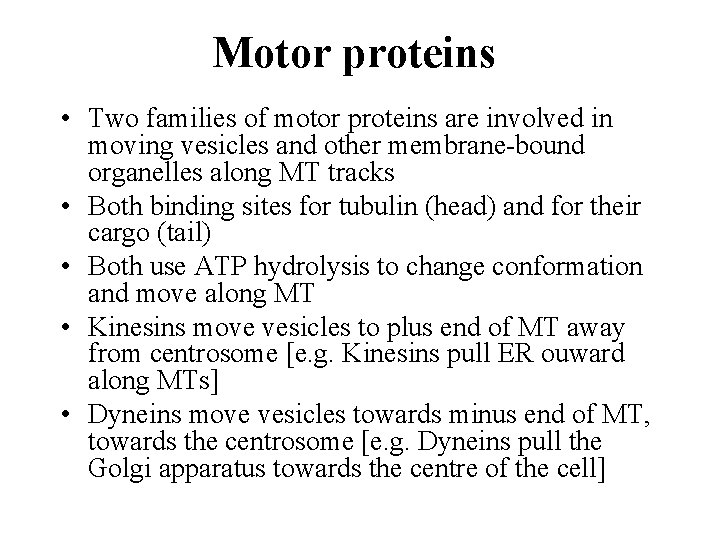
Motor proteins • Two families of motor proteins are involved in moving vesicles and other membrane-bound organelles along MT tracks • Both binding sites for tubulin (head) and for their cargo (tail) • Both use ATP hydrolysis to change conformation and move along MT • Kinesins move vesicles to plus end of MT away from centrosome [e. g. Kinesins pull ER ouward along MTs] • Dyneins move vesicles towards minus end of MT, towards the centrosome [e. g. Dyneins pull the Golgi apparatus towards the centre of the cell]
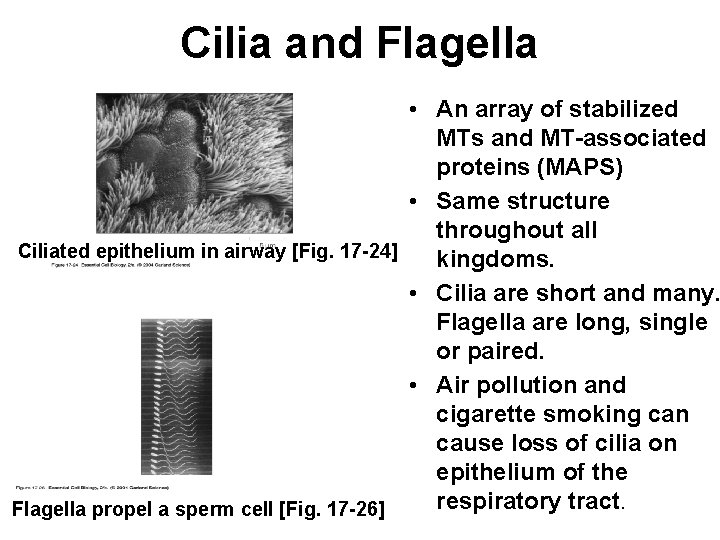
Cilia and Flagella • An array of stabilized MTs and MT-associated proteins (MAPS) • Same structure throughout all Ciliated epithelium in airway [Fig. 17 -24] kingdoms. • Cilia are short and many. Flagella are long, single or paired. • Air pollution and cigarette smoking can cause loss of cilia on epithelium of the respiratory tract. Flagella propel a sperm cell [Fig. 17 -26]
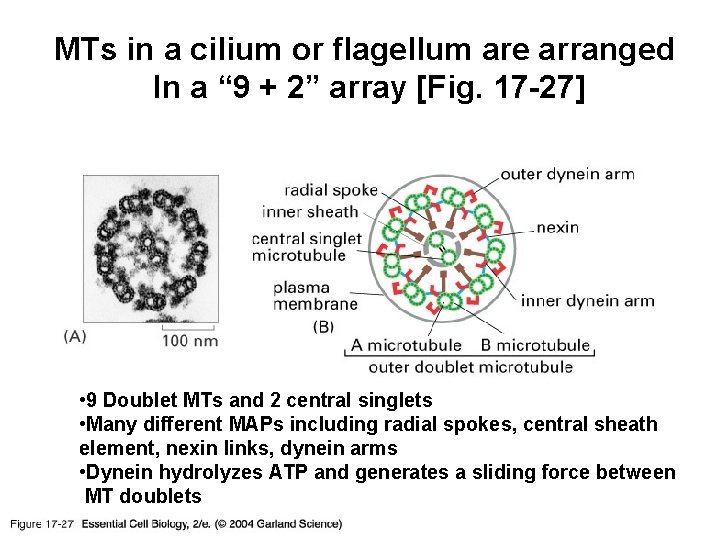
MTs in a cilium or flagellum are arranged In a “ 9 + 2” array [Fig. 17 -27] 17_27_9_+_2_array. jpg • 9 Doublet MTs and 2 central singlets • Many different MAPs including radial spokes, central sheath element, nexin links, dynein arms • Dynein hydrolyzes ATP and generates a sliding force between MT doublets
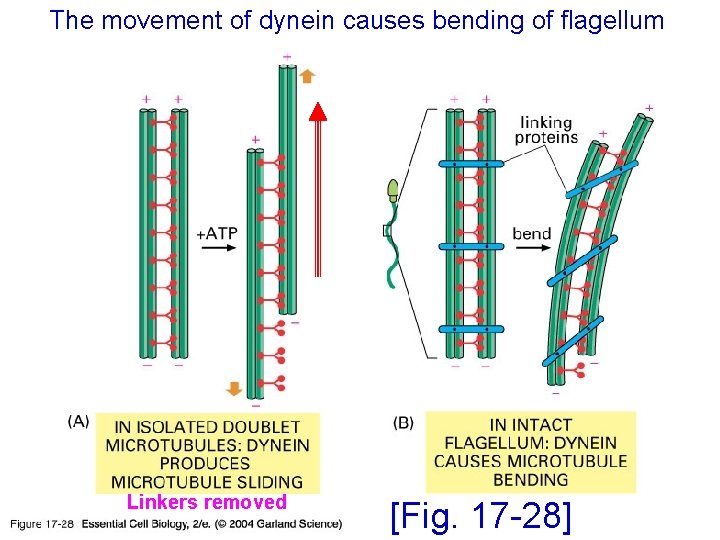
The movement of dynein causes bending of flagellum 17_28_dynein_flagell. jpg Linkers removed [Fig. 17 -28]
![Actin Filaments [Fig. 17 -2] 17_02_03_protein_filament. jpg Actin Filaments [Fig. 17 -2] 17_02_03_protein_filament. jpg](http://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-32.jpg)
Actin Filaments [Fig. 17 -2] 17_02_03_protein_filament. jpg
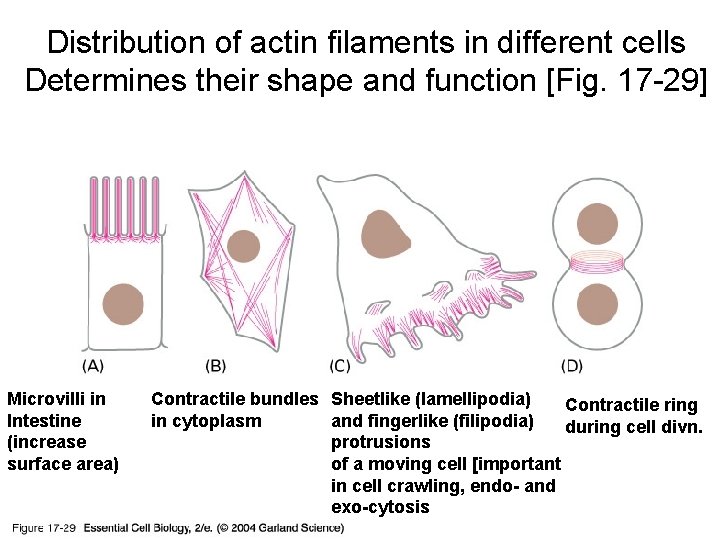
Distribution of actin filaments in different cells Determines their shape and function [Fig. 17 -29] 17_29_Actin_filaments. jpg Microvilli in Intestine (increase surface area) Contractile bundles Sheetlike (lamellipodia) Contractile ring in cytoplasm and fingerlike (filipodia) during cell divn. protrusions of a moving cell [important in cell crawling, endo- and exo-cytosis
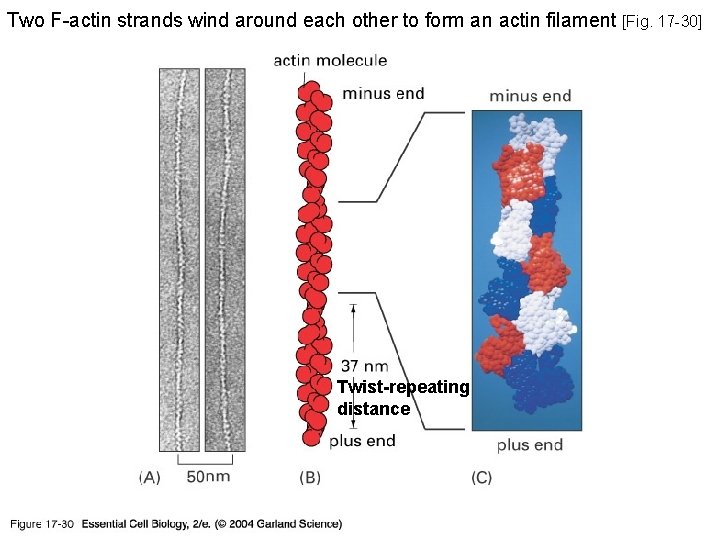
Two F-actin strands wind around each other to form an actin filament [Fig. 17 -30] 17_30_protein threads. jpg Twist-repeating distance
![ATP hydrolysis induce dynamic instability of actin filaments [Fig. 17 -31] Actin with bound ATP hydrolysis induce dynamic instability of actin filaments [Fig. 17 -31] Actin with bound](http://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-35.jpg)
ATP hydrolysis induce dynamic instability of actin filaments [Fig. 17 -31] Actin with bound ADP ATP minus end Phalloidin: A cyclic peptide from the death cap fungus, Amanita phalloides, inhibits the depolymerization of actin, thereby stabilizing actin microfilaments plus end Cytochalasin D: A fungal metabolite, Inhibits the polymerization of actin microfilaments
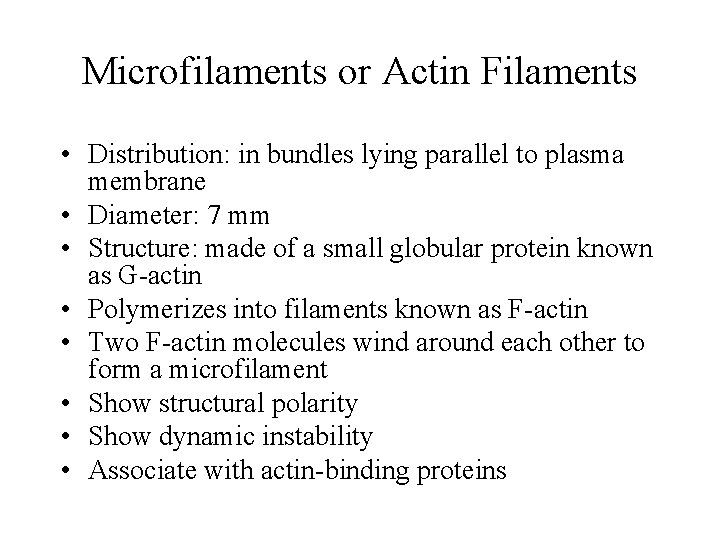
Microfilaments or Actin Filaments • Distribution: in bundles lying parallel to plasma membrane • Diameter: 7 mm • Structure: made of a small globular protein known as G-actin • Polymerizes into filaments known as F-actin • Two F-actin molecules wind around each other to form a microfilament • Show structural polarity • Show dynamic instability • Associate with actin-binding proteins
![Actin-binding proteins regulate the behavior of actin filaments [Fig. 17 -32] 17_32_Actin_binding. jpg (e. Actin-binding proteins regulate the behavior of actin filaments [Fig. 17 -32] 17_32_Actin_binding. jpg (e.](http://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-37.jpg)
Actin-binding proteins regulate the behavior of actin filaments [Fig. 17 -32] 17_32_Actin_binding. jpg (e. g. thymosin and profilin) (e. g. gelsolin)
![Actin polymerization pushes cell edge forward, contraction pulls cell body along [Fig. 17 -33] Actin polymerization pushes cell edge forward, contraction pulls cell body along [Fig. 17 -33]](http://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-38.jpg)
Actin polymerization pushes cell edge forward, contraction pulls cell body along [Fig. 17 -33]
![Actin in amoeboid movement of a fibroblast [Fig. 17 -34] cortex lamellipodia filopodia Actin in amoeboid movement of a fibroblast [Fig. 17 -34] cortex lamellipodia filopodia](http://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-39.jpg)
Actin in amoeboid movement of a fibroblast [Fig. 17 -34] cortex lamellipodia filopodia
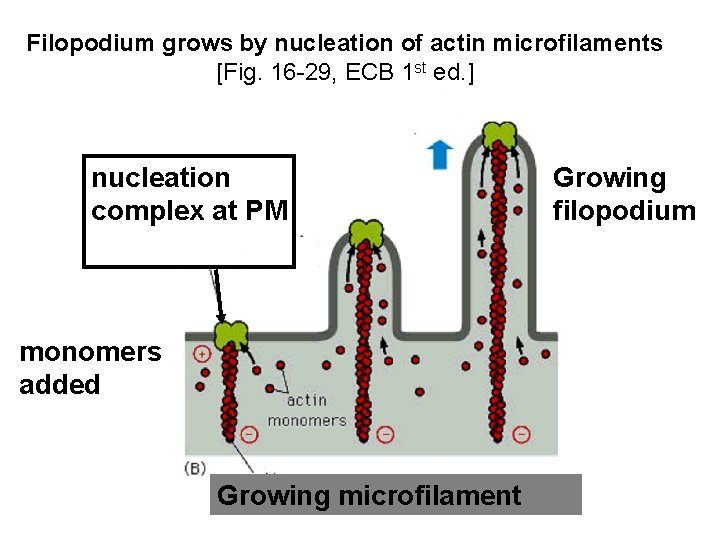
Filopodium grows by nucleation of actin microfilaments [Fig. 16 -29, ECB 1 st ed. ] nucleation complex at PM monomers added Growing microfilament Growing filopodium
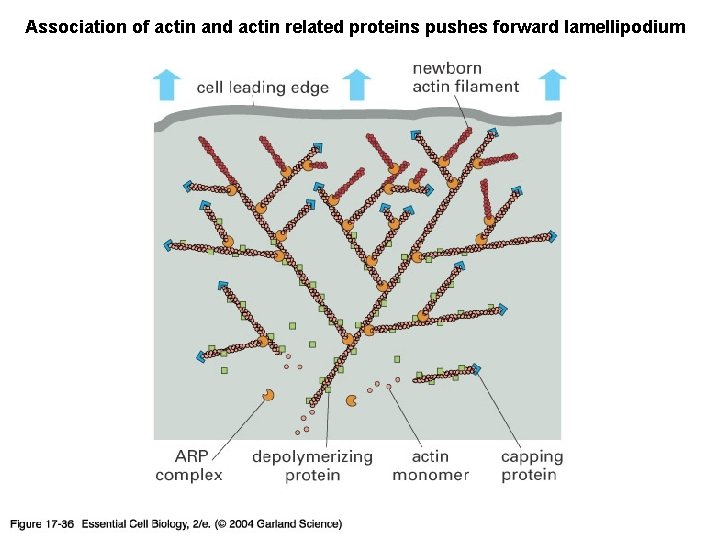
Association of actin and actin related proteins pushes forward lamellipodium 17_36_actin_meshwork. jpg
![Roles of actin-dependent motor protein, myosin I [Fig. 17 -38] The head group of Roles of actin-dependent motor protein, myosin I [Fig. 17 -38] The head group of](http://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-42.jpg)
Roles of actin-dependent motor protein, myosin I [Fig. 17 -38] The head group of myosin I walks towards the plus end of the actin filament. 17_38_myosin_I. jpg Myosin I: Move a vesicle relative to an actin filament. Myosin I: Move an actin filament.
![Myosin-II molecules can associate with one another to form myosin filaments [Fig. 17 -40] Myosin-II molecules can associate with one another to form myosin filaments [Fig. 17 -40]](http://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-43.jpg)
Myosin-II molecules can associate with one another to form myosin filaments [Fig. 17 -40] 17_40_Myosin_II. jpg [Coiled-coil] Tails Bipolar myosin filament
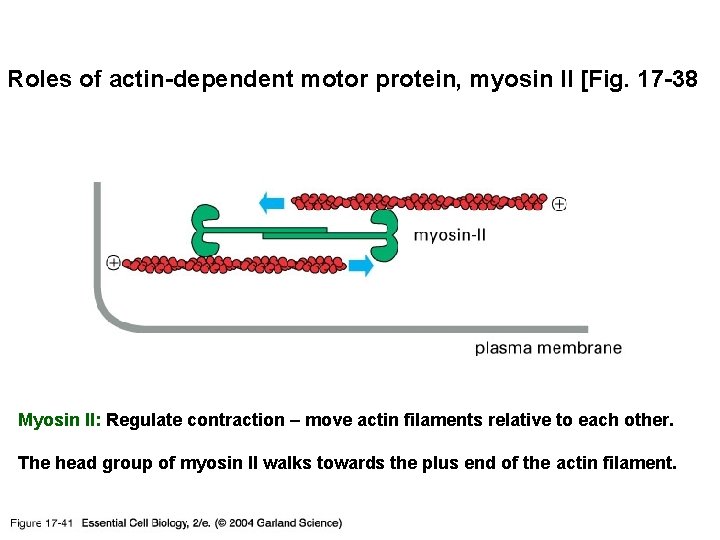
Roles of actin-dependent motor protein, myosin II [Fig. 17 -38 17_41_slide_actin. jpg Myosin II: Regulate contraction – move actin filaments relative to each other. The head group of myosin II walks towards the plus end of the actin filament.
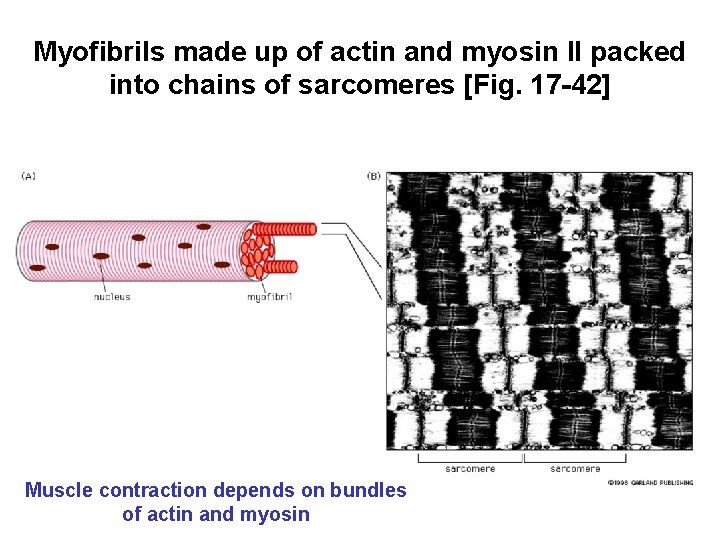
Myofibrils made up of actin and myosin II packed into chains of sarcomeres [Fig. 17 -42] Muscle contraction depends on bundles of actin and myosin
![Sarcomeres (contractile units of muscle) are arrays of actin and myosin [Fig. 17 -43] Sarcomeres (contractile units of muscle) are arrays of actin and myosin [Fig. 17 -43]](http://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-46.jpg)
Sarcomeres (contractile units of muscle) are arrays of actin and myosin [Fig. 17 -43] Z disc: attachment points For actin filaments
![Muscles contract by a sliding-filament mechanism [Fig. 17 -44] 17_44_Muscles contract. jpg + + Muscles contract by a sliding-filament mechanism [Fig. 17 -44] 17_44_Muscles contract. jpg + +](http://slidetodoc.com/presentation_image/273bc44fae9f8ce6f97745523cee3f0c/image-47.jpg)
Muscles contract by a sliding-filament mechanism [Fig. 17 -44] 17_44_Muscles contract. jpg + + The myosin heads walk toward the plus end of the adjacent actin filament driving a sliding motion during muscle contraction.
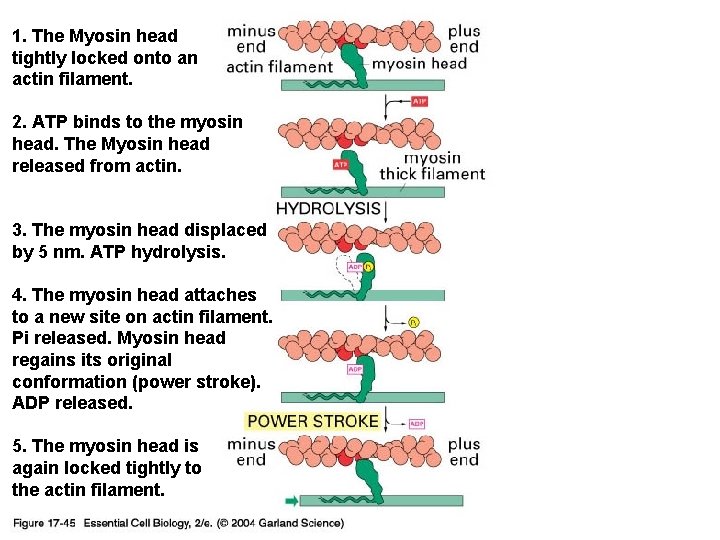
1. The Myosin head tightly locked onto an actin filament. 17_45_myosin_walks. jpg 2. ATP binds to the myosin head. The Myosin head released from actin. 3. The myosin head displaced by 5 nm. ATP hydrolysis. 4. The myosin head attaches to a new site on actin filament. Pi released. Myosin head regains its original conformation (power stroke). ADP released. 5. The myosin head is again locked tightly to the actin filament.

Experimental Methodology, Techniques and Approaches for Studying the Cytoskeleton 1. Modern microscopy techniques 2. Drugs and mutations to disrupt cytoskeletal structures
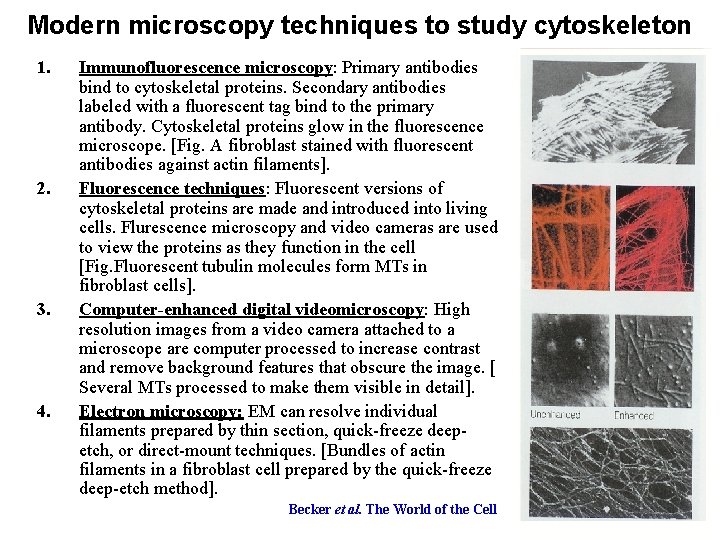
Modern microscopy techniques to study cytoskeleton 1. 2. 3. 4. Immunofluorescence microscopy: Primary antibodies bind to cytoskeletal proteins. Secondary antibodies labeled with a fluorescent tag bind to the primary antibody. Cytoskeletal proteins glow in the fluorescence microscope. [Fig. A fibroblast stained with fluorescent antibodies against actin filaments]. Fluorescence techniques: Fluorescent versions of cytoskeletal proteins are made and introduced into living cells. Flurescence microscopy and video cameras are used to view the proteins as they function in the cell [Fig. Fluorescent tubulin molecules form MTs in fibroblast cells]. Computer-enhanced digital videomicroscopy: High resolution images from a video camera attached to a microscope are computer processed to increase contrast and remove background features that obscure the image. [ Several MTs processed to make them visible in detail]. Electron microscopy: EM can resolve individual filaments prepared by thin section, quick-freeze deepetch, or direct-mount techniques. [Bundles of actin filaments in a fibroblast cell prepared by the quick-freeze deep-etch method]. Becker et al. The World of the Cell
Drug Treatments 1. Colchicine: An alkaloid from the Autumn crocus, Colchicum autumnale). Binds to tubulin monomers and prevents polymerization in MTs. 2. Taxol: from the Pacific Yew tree, Taxus brevifolis binds tightly to MTs and stabilizes them. It prevents MTs from dissociating. 3. Cytochalasin D: A fungal metabolite, inhibits the polymerization of actin microfilaments. 4. Phalloidin: A cyclic peptide from the death cap fungus, Amanita phalloides, inhibits the depolymerization of actin, thereby stabilizing actin microfilaments
- Slides: 51