BIOGEOCHEMICAL CYCLES WHAT IS BIOGEOCHEMICAL CYCLING Bio is
BIOGEOCHEMICAL CYCLES
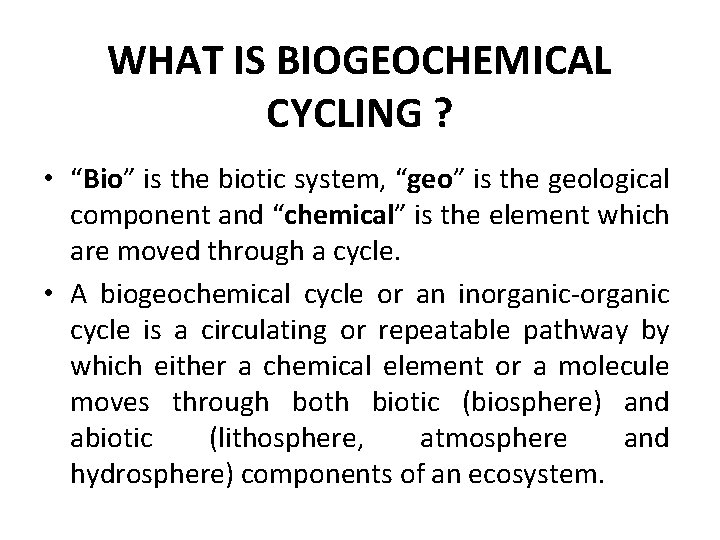
WHAT IS BIOGEOCHEMICAL CYCLING ? • “Bio” is the biotic system, “geo” is the geological component and “chemical” is the element which are moved through a cycle. • A biogeochemical cycle or an inorganic-organic cycle is a circulating or repeatable pathway by which either a chemical element or a molecule moves through both biotic (biosphere) and abiotic (lithosphere, atmosphere and hydrosphere) components of an ecosystem.

• The main chemical elements that are cycled are : carbon (C), hydrogen (H), nitrogen (N), oxygen (O), phosphorous (P) and sulfur (S). • These are the building blocks of the life and are used for essential processes, such as metabolism, the formation of amino acids, cell respiration and building of tissues. • At particular stages of their cycling, any of the elements may be stored and accumulated within a particular place for a long period time (e. g. within a rocky substrate or in the atmosphere). These places are called “sinks” or “reservoirs”.
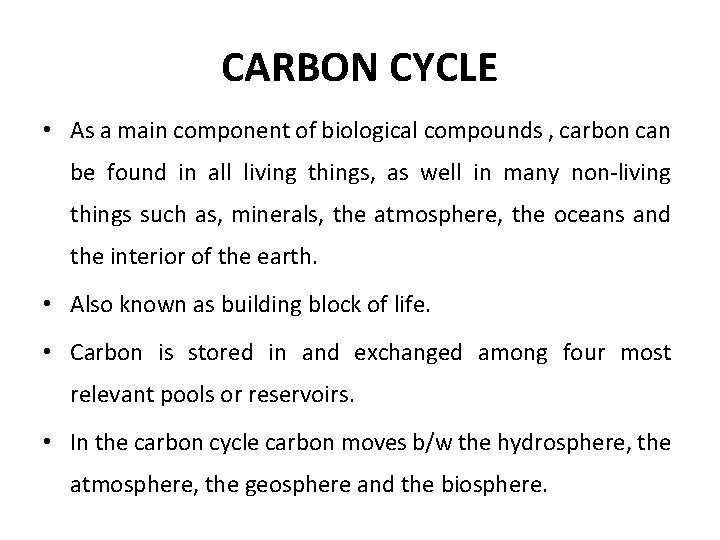
CARBON CYCLE • As a main component of biological compounds , carbon can be found in all living things, as well in many non-living things such as, minerals, the atmosphere, the oceans and the interior of the earth. • Also known as building block of life. • Carbon is stored in and exchanged among four most relevant pools or reservoirs. • In the carbon cycle carbon moves b/w the hydrosphere, the atmosphere, the geosphere and the biosphere.

• In the non living pools carbon is stored as carbonate (Ca. CO 3), rocks dead organic matter, such as humus in soil. • It may find in the form of carbon dioxide (CO 2), carbon monoxide (CO). CO 2 dissolved in water to form HCO 3. • Where CO 2 is a green house gas and traps heat in the atmosphere. Without it and other green house gases , Earth would be a frozen world. • The recent increase in amounts of greenhouse gases such as CO 2 having a significant impact on the warming of our planet. • By following the carbon cycle we can also study the flow of energy needed for life is stored between carbon molecules in organic matter as proteins and fats.
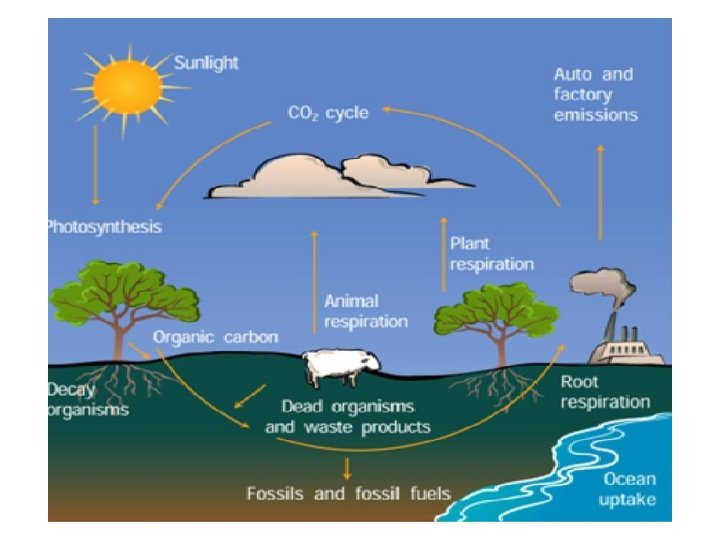

• Carbon moves from the atmosphere to plants where it exist as CO 2. Through the process of photosynthesis, CO 2 is pulled from the air to produce food made from carbon for plant growth. • Carbon moves from plants to animals through food chain. Animals that eat other animals get the carbon from their food too. • From plants and animals carbon moves to the soils. When they die, the decomposition bring the carbon into ground. Some is buried and will become fossil fuel in millions and millions of year.

• Carbon moves from fossil fuels to the atmosphere when fuels are burned. When humans burn fossil fuels to power factories, power plants, cars and trucks, most of the carbon quickly enters the atmosphere as CO 2. • Each year five & a half billion tons of carbon is released by burning fossil fuels. Of this massive amount, 3. 3 billion tons stays in the atmosphere. Most of the remainder becomes dissolved in seawater. • Carbon moves from the atmosphere to the oceans. The oceans, and other bodies of water, absorb some carbon from the atmosphere. Thus carbon is dissolved into the water.

v. MICROBIAL DEGRADATION OF CELLULOSE : • Cellulose is the most abundant organic matter in nature. • It is a polysaccharide composed of glucose molecules linked together in a linear chain of 1 -4 glycosidic linkage. • Several micro-organisms are capable of degrading cellulose : Ø Fungi : Aspergillus, Fusarium, Trichoderma, Curvularia. Ø Bacteria : Bacillus, Achromobacter, Pseudomonas, Vibrio, Nocardia, Cellulomonas, Streptomyces.
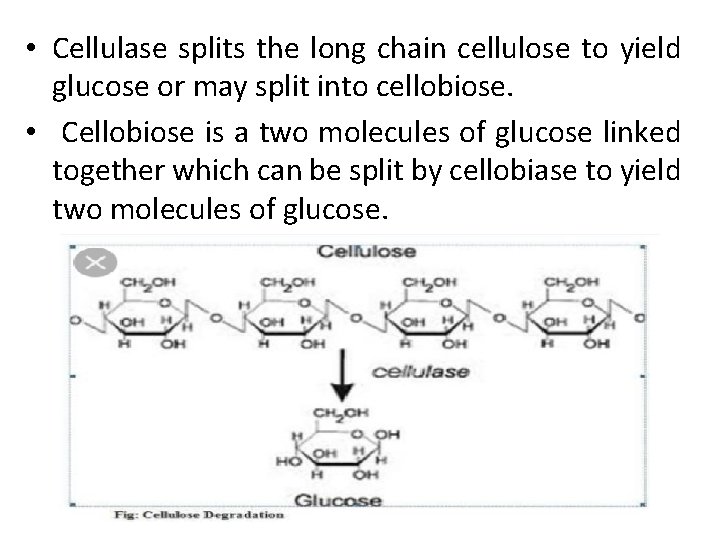
• Cellulase splits the long chain cellulose to yield glucose or may split into cellobiose. • Cellobiose is a two molecules of glucose linked together which can be split by cellobiase to yield two molecules of glucose.

v. MICROBIAL DEGRADATION OF HEMICELLULOSE : • Hemicellulose are water soluble polysaccharides and consists of hexoses, pentoses and uronic acids. • Glucose, galactose, mannose, xylose, arabinose, glucoronic acid and galactouronic acids are commonly found in hemicellulose plants. • The molecules contains aromatic ring as building blocks. • Hemicellulase degrade the hemicellulose and release constituent components.
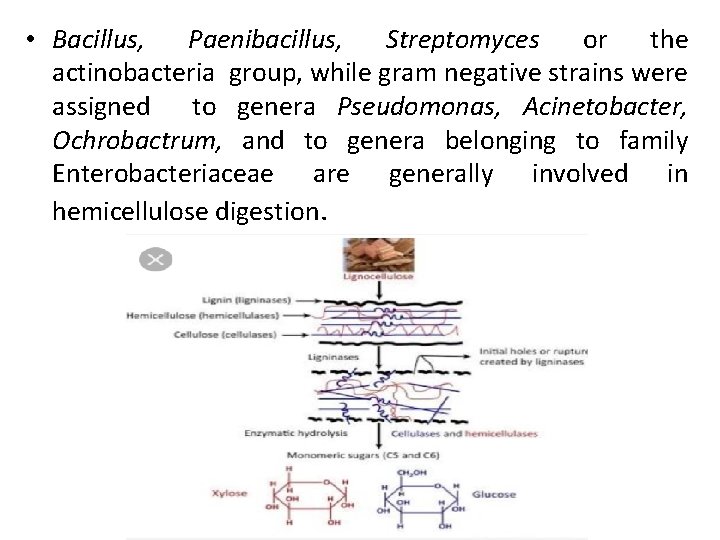
• Bacillus, Paenibacillus, Streptomyces or the actinobacteria group, while gram negative strains were assigned to genera Pseudomonas, Acinetobacter, Ochrobactrum, and to genera belonging to family Enterobacteriaceae are generally involved in hemicellulose digestion.
v MICROBIAL DEGRADATION OF LIGNIN : • It is one of the most resistant organic substances for the micro-organisms to degrade. • Many Basidiomycetes have been found to possess special capacity to degrade the lignin. • Only rarely bacteria have been found to reduce lignin. The fungi commonly found are : Fomes , Ganoderma, Agaricus, Armilaria, Polyporous, etc. • These fungi utilizes high lignin containing materials. • The enzymatic breakdown of lignin may result in simpler aromatic compound syringaldehyde, vanillin, phydroxy-benzaldehyde, and ferulic acid.

• Final cleavage of aromatic ring may take place with involvement of other fungi and bacteria to yield low molecular weight organic acids, carbondioxide, methane etc. • Fungi degrade lignin by secreting enzymes collectively in termed “ligninases”. They can be classified as either phenol oxidases (laccase) or heme peroxidases , lignin peroxidases (Li. P), manganese peroxidase (Mn. P) and versatile peroxidases (VP).
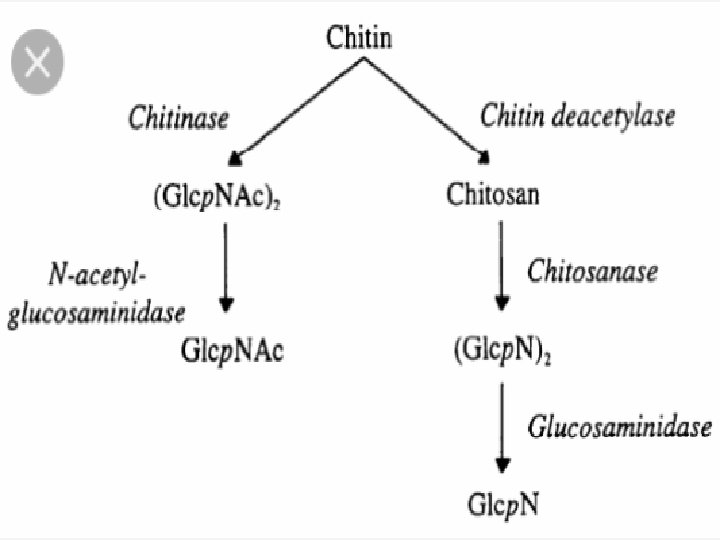
v MICROBIAL DEGRADATION OF CHITIN : • It is a polysaccharide whose basic unit is an amino sugar. • It is a structural component giving mechanical strength to several plants and animals. • It is also present in fungal cell wall and insects. • This is one of the hardiest organic molecules for microbial action in soil. • The organisms involved are : Streptomyces, Nocardia, Micromonospora, Aspergillus, Penicillium, Mucor, Trichoderma, etc. • The breakdown products are : glucosamine, acetic acid, ammonia, carbondioxide. • Chitinase initiates the process to reduce chitin to diacetylchitobiase & chitobiase reduces the latter into acetylglucosamine.

NITROGEN CYCLE • There is a laundry list of elements that animals need for survival. One such element is Nitrogen. But we can’t just get nitrogen from the air. It needs to be converted to nitrates, via process called nitrogen cycle. • Nitrogen cycle consists of four main steps namely : v Nitrogen fixation v Proteolysis v Ammonification / Decay v Nitrification v Denitrification / nitrate reduction.
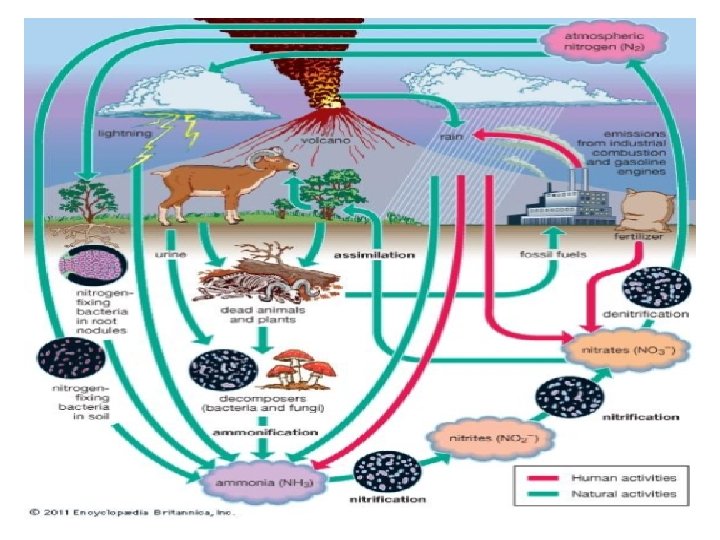

v NITROGEN FIXATION : • • Ø Ø • Nitrogen is most common soil nutrient required by the plants but due to the high stability of triple bond b/w N 2 molecules it become difficult to use it directly. However there are two pathways to make atmospheric nitrogen available to the plants : Through the formation of industrial fertilizer by a chemical process, Nitrogen fixation by microbes i. e. biological nitrogen fixation. Utilization of atmospheric dinitrogen gas (N 2) as a source of cell nitrogen by way of its reduction to ammonia by a biological agent is called biological nitrogen fixation.
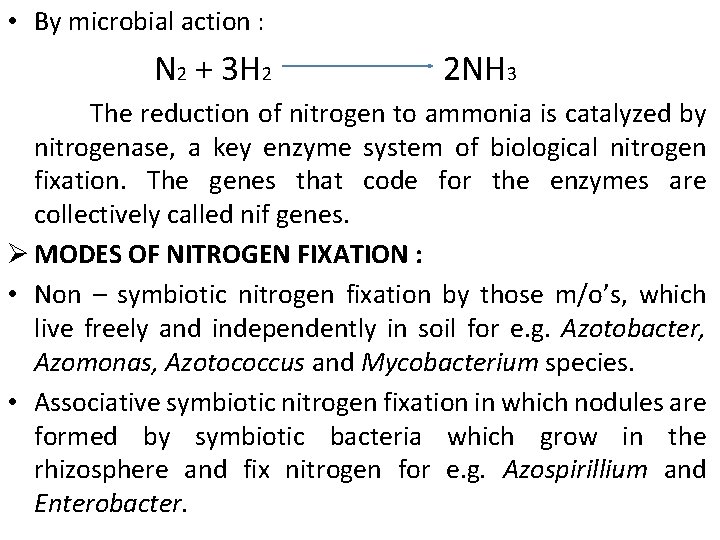
• By microbial action : N 2 + 3 H 2 2 NH 3 The reduction of nitrogen to ammonia is catalyzed by nitrogenase, a key enzyme system of biological nitrogen fixation. The genes that code for the enzymes are collectively called nif genes. Ø MODES OF NITROGEN FIXATION : • Non – symbiotic nitrogen fixation by those m/o’s, which live freely and independently in soil for e. g. Azotobacter, Azomonas, Azotococcus and Mycobacterium species. • Associative symbiotic nitrogen fixation in which nodules are formed by symbiotic bacteria which grow in the rhizosphere and fix nitrogen for e. g. Azospirillium and Enterobacter.

Ø Symbiotic Nitrogen Fixation : • Through nodule formation in leguminous plants like in clover, soybeans, peas, etc. by bacteria like Rhizobium, Azorhizobium, Mesorhizobium, etc. Legume rhizobium nitrogen fixation is of considerable agricultural significance as it leads to a greater quantity of combined nitrogen in the soil. • Through nodule formation in non – leguminous plants for e. g. the best known plant is alder (Alnus spp. ), the bacterium involved in nodule formation in this case is an Actinomycete & Frankia spp. • Non – nodulation nitrogen fixing associations are formed b/w cyanobacteria and various plants for e. g. the cyanobacterium Anabaena azollae forms symbiotic association with Azolla plant.
v PROTEOLYSIS : • Plants use the ammonia produced by symbiotic and non-symbiotic nitrogen fixation to make their amino acids and eventually plant proteins. • Animals eat the plants and convert plant proteins into animal proteins. • Upon death, plants and animals undergo microbial decay in the soil and the nitrogen contained in their proteins is released. • Thus the process of enzymatic breakdown of proteins by the microorganisms with the help of proteolysis enzymes is known as “Proteolysis”.
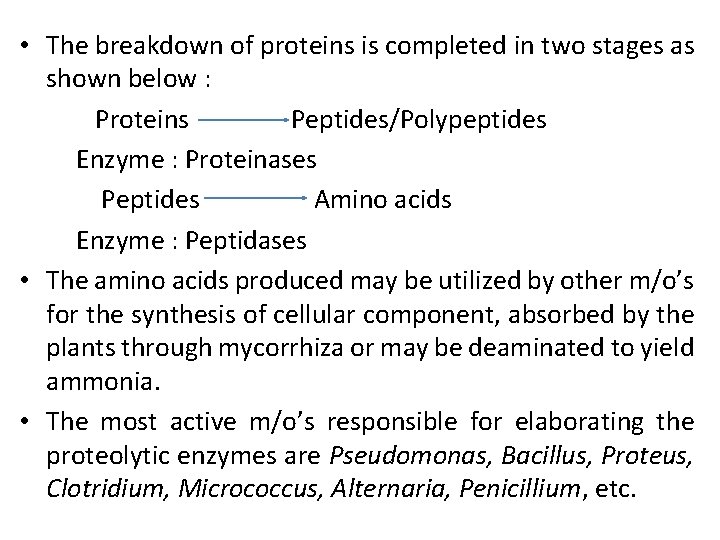
• The breakdown of proteins is completed in two stages as shown below : Proteins Peptides/Polypeptides Enzyme : Proteinases Peptides Amino acids Enzyme : Peptidases • The amino acids produced may be utilized by other m/o’s for the synthesis of cellular component, absorbed by the plants through mycorrhiza or may be deaminated to yield ammonia. • The most active m/o’s responsible for elaborating the proteolytic enzymes are Pseudomonas, Bacillus, Proteus, Clotridium, Micrococcus, Alternaria, Penicillium, etc.
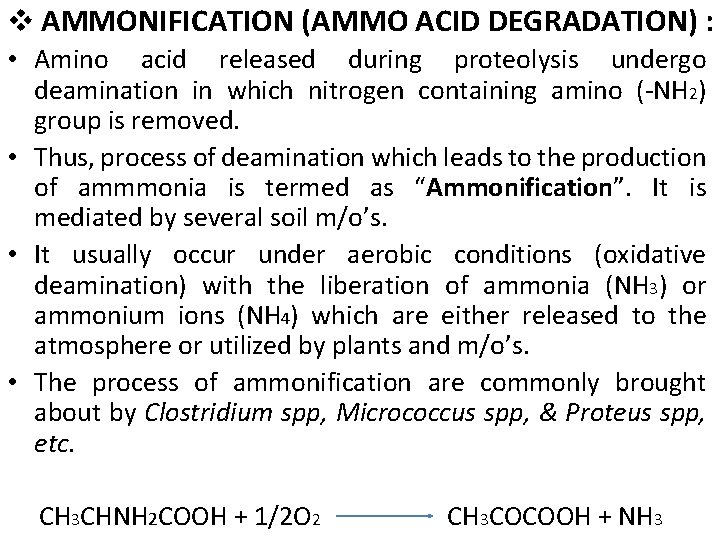
v AMMONIFICATION (AMMO ACID DEGRADATION) : • Amino acid released during proteolysis undergo deamination in which nitrogen containing amino (-NH 2) group is removed. • Thus, process of deamination which leads to the production of ammmonia is termed as “Ammonification”. It is mediated by several soil m/o’s. • It usually occur under aerobic conditions (oxidative deamination) with the liberation of ammonia (NH 3) or ammonium ions (NH 4) which are either released to the atmosphere or utilized by plants and m/o’s. • The process of ammonification are commonly brought about by Clostridium spp, Micrococcus spp, & Proteus spp, etc. CH 3 CHNH 2 COOH + 1/2 O 2 CH 3 COCOOH + NH 3
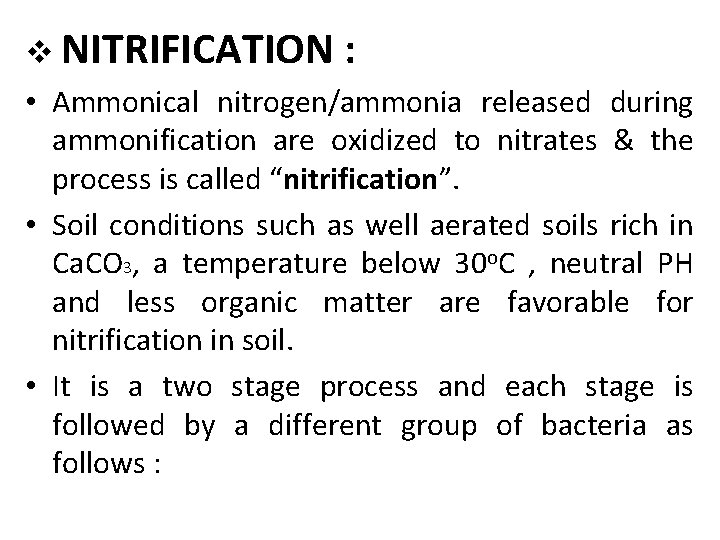
v NITRIFICATION : • Ammonical nitrogen/ammonia released during ammonification are oxidized to nitrates & the process is called “nitrification”. • Soil conditions such as well aerated soils rich in Ca. CO 3, a temperature below 30 o. C , neutral PH and less organic matter are favorable for nitrification in soil. • It is a two stage process and each stage is followed by a different group of bacteria as follows :

Ø STAGE 1 : Oxidation of ammonia of nitrite is brought about by ammonia oxidizing bacteria for e. g. Nitrosomonas europaea, Nitrosococcus nitrosus, Nitrosospira briensis, Nitrosovibrio & Nitrocystis & the process is known as nitrosification. The reaction is presented as follow: 2 NH 3 + 1/2 O 2 NO 2 + 2 H + H 2 O Ø STAGE 2 : In the second step nitrite is oxidized to nitrate by nitrite oxidizing bacteria such as Nitrobacter winogradsky, Nitospira gracilis, Nitrosococcus mobiis etc. and several fungi (e. g. penicillium, Aspergillus) and actinomycetes (Streptomyces, Nocardia). NO 2 (-) + 1/2 O 2 NO 3

• The nitrate thus formed may be utilized by the microorganisms, assimilated by plants, reduced to nitrite and ammonia or nitrogen gas or lost through leaching depending on soil conditions. • The nitrifying bacteria (ammonia oxidizer and nitrite oxidizer) are aerobic gram negative and chemoautotrophic and are the common inhabitants of soil, sewage and aquatic environment.
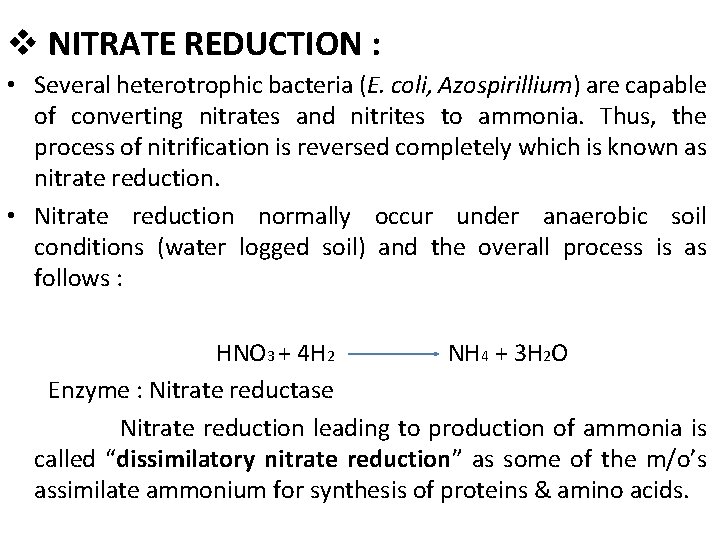
v NITRATE REDUCTION : • Several heterotrophic bacteria (E. coli, Azospirillium) are capable of converting nitrates and nitrites to ammonia. Thus, the process of nitrification is reversed completely which is known as nitrate reduction. • Nitrate reduction normally occur under anaerobic soil conditions (water logged soil) and the overall process is as follows : HNO 3 + 4 H 2 NH 4 + 3 H 2 O Enzyme : Nitrate reductase Nitrate reduction leading to production of ammonia is called “dissimilatory nitrate reduction” as some of the m/o’s assimilate ammonium for synthesis of proteins & amino acids.
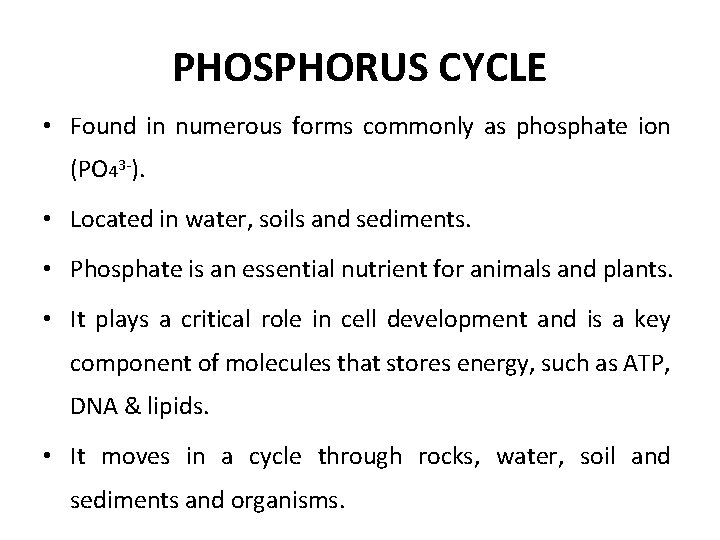
PHOSPHORUS CYCLE • Found in numerous forms commonly as phosphate ion (PO 43 -). • Located in water, soils and sediments. • Phosphate is an essential nutrient for animals and plants. • It plays a critical role in cell development and is a key component of molecules that stores energy, such as ATP, DNA & lipids. • It moves in a cycle through rocks, water, soil and sediments and organisms.
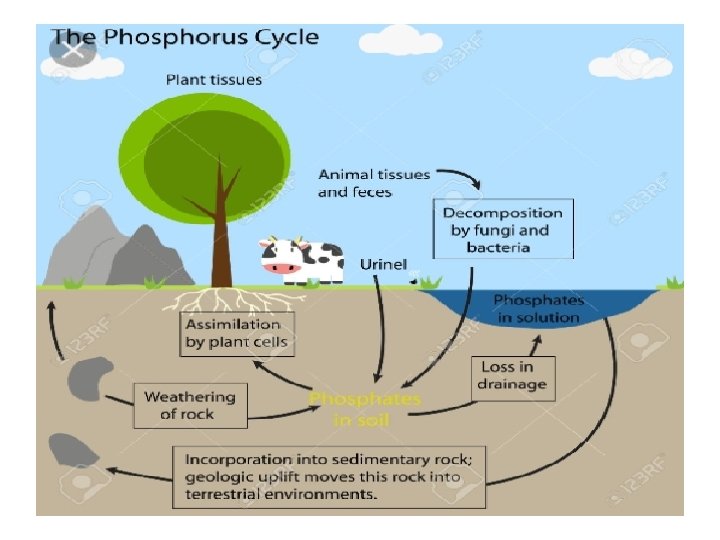

v KEY STEPS IN PHOSPHORUS CYCLE : • Overtime, rain and weathering cause rocks to release phosphate ions and other minerals. This inorganic phosphate is then distributed in soils and water. • Plants take up phosphate from soil. Plants may be consumed by animals & upon their death & decomposition organic phosphate from them is returned to the soil. • Within the soil, organic forms of phosphate can be made available to plants by bacteria that breakdown organic matter to inorganic forms of phosphorus. This process is known as mineralisation. • Phosphorus in soil can end up in waterways and eventually oceans. Once there, it can be incorporated into sediments overtime.
• The immobilization of inorganic phosphate, in contrast, is the reverse reaction of mineralization. During immobilization, m/o’s convert inorganic forms to organic phosphate, which are then incorporated into their living cells. • Mineralization and immobilization of phosphorus occur simultaneously in soil. • The factors like temperature, moisture and aeration can effect the phosphate mineralization and immobilization. • In an average soil, approximately 50% if total phosphorus is organic. Thus, it is the very important aspect of the P cycle.
SULFUR CYCLE • Sulfur is the most abundant and widely distributed element in the nature. • It is found in the free and combined state. • In the soil, sulfur is in the organic form which is metabolized by soil m/o’s to make it available in an inorganic form for plant nutrition. • Various transformations of the sulfur in soil results mainly due to microbial activity. • Major types of transformations involved in the cycling of sulfur are Minerlization, Immobilization, Oxidation and Reduction.
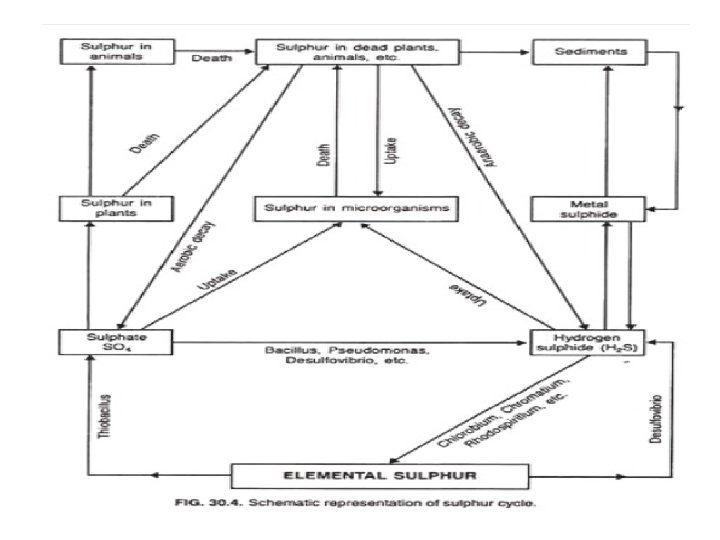
v MINERALIZATION : • The breakdown or decomposition of large organic sulfur units into smaller units and their conversion into inorganic compounds (sulfates) by the m/o’s. • The rate of sulfur mineralization is about 1 -10 % per year. v IMMOBILIZATION : • Microbial conversion of inorganic sulfur compounds to organic sulfur compounds. v OXIDATION : • Oxidation of elemental sulfur and inorganic sulfur compounds such as H 2 S, sulphite & thiosulphite to sulfate (SO 4) is brought about by chemoautotrophic and photosynthetic bacteria.
• Sulfur released from degraded plants and animal proteins is then oxidized to sulfates in the presence of oxygen. • Under anaerobic conditions (water logged soils) organic sulfur is decomposed to produce hydrogen sulfide (H 2 S). • H 2 S can also accumulate during the reduction of sulfates under anaerobic conditions which can be further oxidized to sulfates under aerobic conditions. The reactions involve as follow : Ø 2 S + 3 O 2 + 2 H 2 O Ø CO 2 + 2 H 2 SO 4 2 H + SO 4 (CH 2 O) + H 2 O + 2 S OR H 2 + S + 2 CO 2 + H 2 O H 2 SO 4 + 2(CH 2 O)

• The member of the genus Thiobacillus (obligate chemolithotrophic, non – photosynthetic) e. g. T. ferrooxidans and T. thiooxidans are the main organisms involved in the oxidation of elemental sulfur to sulfates. • Other than Thiobacillus, heterotrophic bacteria (Bacillus, Pseudomonas and Arthrobacter) and fungi (Aspergillus, Penicillium) and some actinomycetes are also reported to oxidize sulfur compounds. • Green and purple bacteria of genera Chlorbium, Chromatium, Rhodopseudomonas are also reported to oxidize sulfur in aquatic environment. • H 2 SO 4 produced during oxidation of sulfur is of great significance in reducing the ph of alkaline soils and in controlling potato scab and rot diseases caused by Streptomyces bacteria.
v REDUCTION OF SULFATE : • Sulfate in the soil is assimilated by plants and m/o’s and incorporated in proteins. This is known as “assimilatory sulfate reduction”. • Sulfate can be reduced to H 2 S by sulfate reducing bacteria (e. g. Desulfovibrio and Desulfatomaculum) and may diminish the availability of sulfur for nutrition. • Dissimilatory sulfate – reduction is favored by the alkaline and anaerobic conditions of soil and sulfates are reduced to hydrogen sulfide. • For e. g. calcium sulfate is attacked under anaerobic condition by the members of the genus Desulfovibrio & Desulfatomaculum to release H 2 S. Ca. SO 4 + 4 H 2 Ca(OH)2 + H 2 S + H 2 O
• H 2 S produced by the reduction of sulfate and sulfur containing amino acid decomposition is further oxidized by some species of green and purple phototrophic bacteria(e. g. Chlorobium, Chromatium) to release elemental sulfur. CO 2 + 2 H 2 + H 2 S (CH 2 O) + H 2 O + 2 S • Desulfovibrio desulficans are non – spore forming, obligate anaerobes that reduce sulfates at rapid rate in waterlogged/flooded soils. • Species of Desulfatomaculum are spore forming, thermophilic obligate anaerobes that reduce sulfate in dry land soils. • All sulfate reducing bacteria excrete an enzyme called “desulfurases” or “bisulphate reductase”. • Rate of sulfate reduction in nature is enhanced by increasing water levels, high temperature and organic matter.
IRON CYCLE • Iron exist in nature either as ferrous ( Fe++) or ferric ( Fe+++) ions. • Ferrous iron is oxidized to spontaneously to ferric state, forming highly insoluble ferric hydroxide. • Plants as well as m/o’s require traces of iron. • Soil m/o’s play an important role in the transformations of iron in number of ways such as :
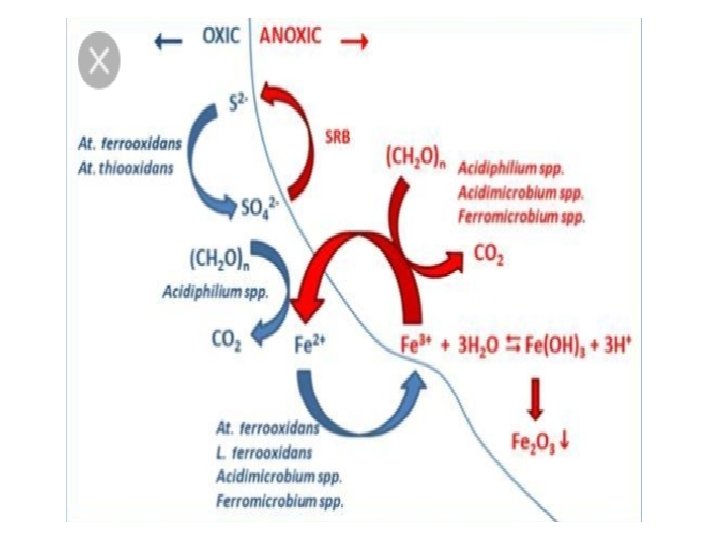

• Thiobacillus ferrooxidans carries out this process under acidic conditions, Gallionella is active under neutral p. H conditions, Sulfolobus functions under acidic, thermophilic conditions. • Sphaerotilus and Leptothrix could oxidize iron and still termed “iron bacteria” by many non-microbiologists. • Iron reduction occur under anaerobic conditions resulting in the accumulation of ferrous ion. • Although many m/o’s can reduce small amounts of iron during their metabolism for e. g. Geobacter metallireducens, G. sulfurreducens, Ferribacterium limneticum and Shewanella putrefaciens.
• Some magnetotactic bacteria such as Aquaspirillum magnetotacticum also help in reduction. • Some bacteria are capable of reducing ferric iron to ferrous which lowers the oxidation-reduction potential of the environment for e. g. Bacillus, Clostridium, Klebsiella etc. • Some chemoautotrophic iron and sulfur bacteria such as Thiobacillus ferroxidans can oxidize ferrous iron to ferric hydroxide which accumulates around the cells. • The iron binding or chelating compounds/ligands produced by m/o’s are called “siderophores”. • VAM has been reported to increase uptake of iron.
MN CYCLE

THANKS …. .
- Slides: 45