BIOENERGETICS OBJECTIVES 1 Define energy 2 Define the
BIOENERGETICS: OBJECTIVES: 1. Define energy 2. Define the 1 st and 2 nd laws of Thermodynamics, including entropy. 3. Describe photosynthesis and respiration in thermodynamic and redox terms. TASK: 1. Task: A jelly donut contains about 1 x 106 J of energy. A gallon of gasoline contains about 1 x 108 J of energy. How many jelly donuts would provide the same amount of energy as a 20 gallon tank of gasoline? `

ENERGY AND SOCIETY Claim: It is not a coincidence that large-scale human slavery in the Western world ended around the same time fossil fuels were rapidly expanding. A human doing physical work for 8 -hours a day can produce about 5 x 105 J of useful work. A gallon of gasoline produces about 2 x 106 J of useful energy when burned in an engine. How many gallons of gasoline produce the same amount of work as 50 humans working all day?
![SOLUTION (2 x 106 J) [gasoline] / (5 x 105 J) [human] = 4. SOLUTION (2 x 106 J) [gasoline] / (5 x 105 J) [human] = 4.](http://slidetodoc.com/presentation_image/4314428a3dd655582f02ea1860955605/image-3.jpg)
SOLUTION (2 x 106 J) [gasoline] / (5 x 105 J) [human] = 4. � 1 gallon of gasoline produces as much work as 4 humans working all day. 50 [humans] / 4 = 12 ½ Evidence: the same amount of work can be done by 50 day-laborers or about 13 gallons of gasoline.

TAKE-HOME MESSAGE The amount of energy produced by burning fossil fuels is huge in comparison to human labor. Example: You could not power a large TV by riding a bicycle… but coal can.

WHAT IS ENERGY? The ability of something to cause a change in itself or its environment. The ability to do work.

LAWS OF THERMODYNAMICS 1 st Law: Energy can neither be created nor destroyed. 2 nd Law of Thermodynamics: In an isolated system, entropy (disorder) increases � So how do living things remain so organized, and in fact increase the organization in and around themselves? ENERGY. Living things use energy to prevent entropy from destroying them. Living things are “negative entropy machines”
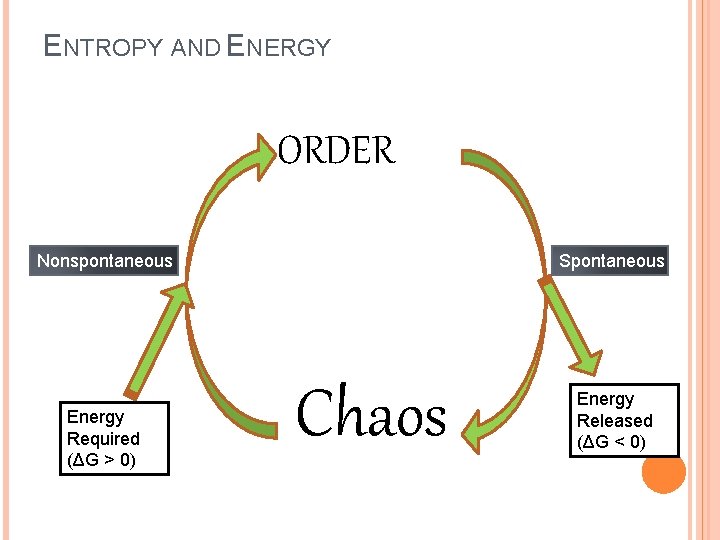
ENTROPY AND ENERGY ORDER Nonspontaneous Energy Required (ΔG > 0) Spontaneous Chaos Energy Released (ΔG < 0)
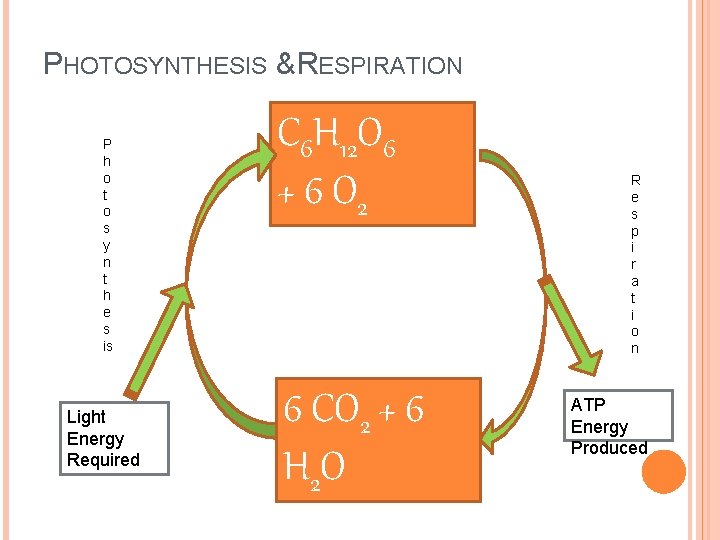
PHOTOSYNTHESIS & RESPIRATION P h o t o s y n t h e s is Light Energy Required C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O R e s p i r a t i o n ATP Energy Produced
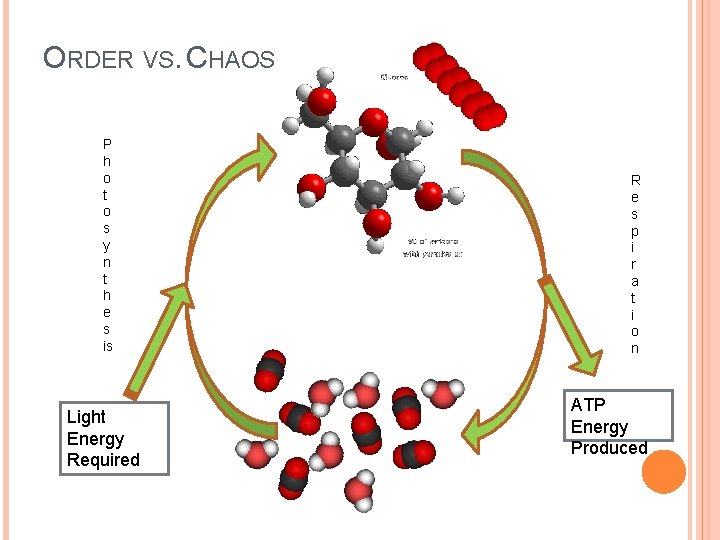
ORDER VS. CHAOS P h o t o s y n t h e s is Light Energy Required R e s p i r a t i o n ATP Energy Produced
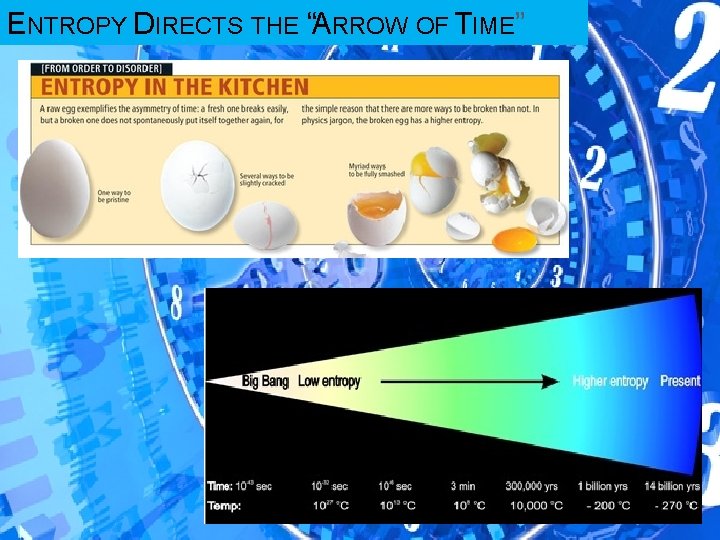
ENTROPY DIRECTS THE “ARROW OF TIME”

… MC Hawking…

KEY BIO-ENERGETIC PROCESSES Photosynthesis : the process by which plants and other photoautotrophs store the energy of light as chemical energy in carbohydrates. (Cellular) Respiration: the process by which animals and other chemotrophic organisms transform chemical energy stored in carbohydrates (or other sources) into available energy (ATP).
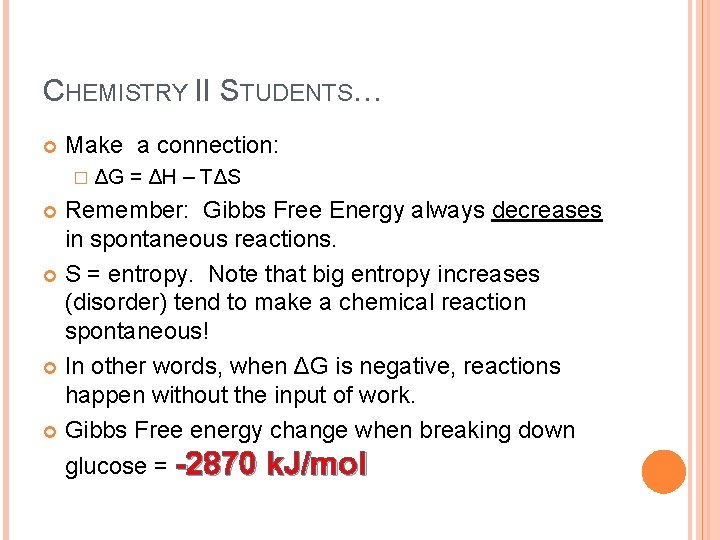
CHEMISTRY II STUDENTS… Make a connection: � ΔG = ΔH – TΔS Remember: Gibbs Free Energy always decreases in spontaneous reactions. S = entropy. Note that big entropy increases (disorder) tend to make a chemical reaction spontaneous! In other words, when ΔG is negative, reactions happen without the input of work. Gibbs Free energy change when breaking down glucose = -2870 k. J/mol

REDOX REACTIONS: IT’S ABOUT ELECTRONS Reducing carbon (adding more H to it) � � � Oxidizing carbon (adding more O) � � � releases energy e. g. combustion, aerobic respiration This releases some of the chemical potential energy stored in the electrons of the chemical system How to remember? � requires energy e. g. photosynthesis. This increases the chemical potential energy stored by electrons in the chemical system L. E. O. the lion says G. E. R. Bottom line? Food is food because it has high-energy electrons you can use to make ATP.
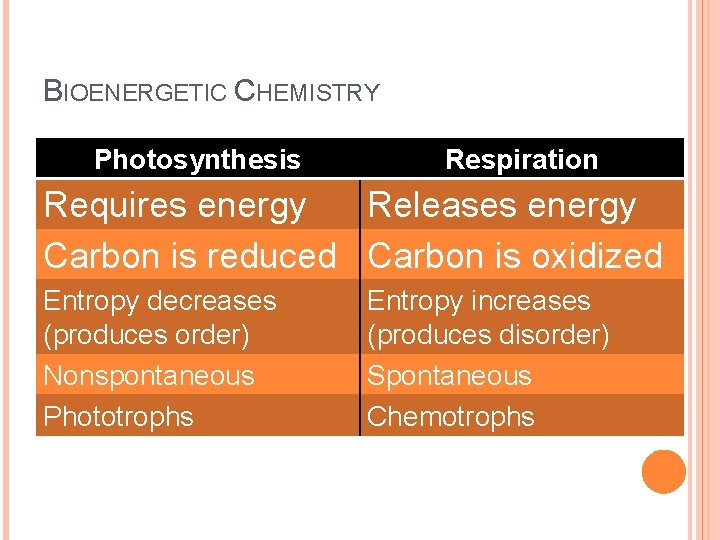
BIOENERGETIC CHEMISTRY Photosynthesis Respiration Requires energy Releases energy Carbon is reduced Carbon is oxidized Entropy decreases (produces order) Nonspontaneous Phototrophs Entropy increases (produces disorder) Spontaneous Chemotrophs
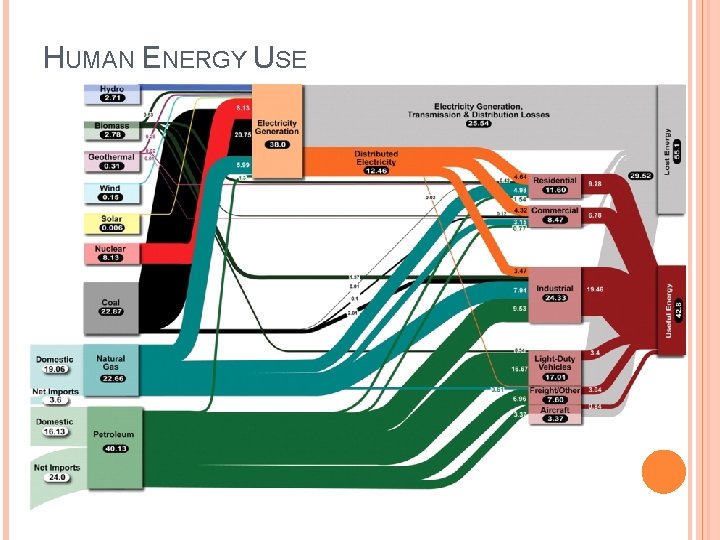
HUMAN ENERGY USE
CONSEQUENCES
- Slides: 17