BIOE 301 Lecture TwentyTwo Schedule Today April 10

BIOE 301 Lecture Twenty-Two

Schedule • Today, April 10 – Lecture 22: FDA Device Regulation & Research Funding • Thursday, April 12 – Lecture 23: Future of Bioengineering in World Health & Review for Final • Tuesday, April 17 – No class! • Thursday, April 19 – Exam 3, Lectures 16 -23 • Tuesday, April 24 – Project Presentations • Final Exam (see syllabus for details)

Reading Assignment for Thursday • Financial Anatomy of Biomedical Research, JAMA. 2005, Sep 21; 294(11): 1333 -42 • Application of Microchip Assay System for the Measurement of C-reactive Protein in Human Saliva, Lab Chip. 2005, 5, 261 -269 • An exam question will draw from these articles

After Today’s Lecture… • Appreciate why it costs so much in the US (time & money) to develop devices and get them to market • Speculate on what this means for developing countries that depend on 1 st world research • Understand the importance and magnitude of research funded by the government
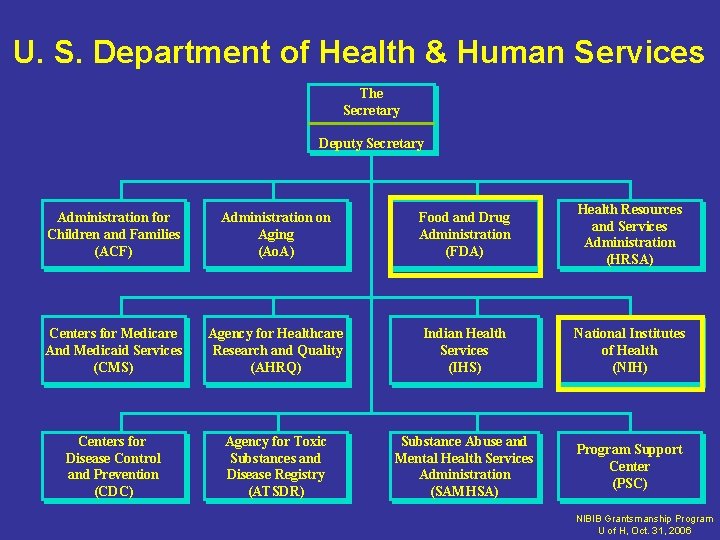
U. S. Department of Health & Human Services The Secretary Deputy Secretary Administration for Children and Families (ACF) Administration on Aging (Ao. A) Food and Drug Administration (FDA) Health Resources and Services Administration (HRSA) Centers for Medicare And Medicaid Services (CMS) Agency for Healthcare Research and Quality (AHRQ) Indian Health Services (IHS) National Institutes of Health (NIH) Centers for Disease Control and Prevention (CDC) Agency for Toxic Substances and Disease Registry (ATSDR) Substance Abuse and Mental Health Services Administration (SAMHSA) Program Support Center (PSC) NIBIB Grantsmanship Program U of H, Oct. 31, 2006
http: //www. fda. gov/oc/orgcharts/fda. pdf

Workings of the FDA • • Responsibility to protect the public and dealing with technological changes Regulates anything for diagnosis, cure, mitigation, or prevention of disease as well as anything intended to affect structure/function of the human body 3 Branches – Drugs – Devices – Biologics – Combination products overseen by panel from three areas Requires the expertise of many - Engineers (including biomedical electrical, and materials) - Biologists and microbiologists - Physcians and other clinicians - Chemists, biochemists, and toxicologists - Medical technologists - Statisticians - Consumer safety officers and field investigators - Human factors specialists

Role of Center for Devices and Radiological Health (CDRH) • Ensure that products coming to market have more benefit than risk • Ensure that products are labeled so that practitioners and patients know what to expect from their use • Regulates 1, 700 types of devices • 23, 000 registered manufacturers • In 1996 received 20, 236 device related submissions

Regulation of Medical Devices • FDA did not regulate devices before 1938 • 1938: – FDA could only challenge sale of products it believed were unsafe – Could only remove them from the market after patient injuries • 1960 s: – Rapid innovation in medical technology – Tried to regulate many as drugs, i. e. contact lenses, IUDs – Catastrophic failures of heart valves and pacemakers • 1970 s: – Broad recognition that different rules were needed to regulate devices – No single policy would work for all devices • Tongue depressor • Artificial heart • Review commission determined that more than 700 deaths and 10, 000 injuries were associated with medical devices

Medical Device Act (1976) • Amendments to FD&C act allowed for classification of medical devices, device listing, establishment registration, adherence to Good Manufacturing Practices (GMP), and extensive control over market introduction of medical devices • Major amendments again in ’ 90, ’ 97, and ’ 02 • However, CDRH can not specifically regulate biomaterials; biomaterials are indirectly regulated according to intended use

Class Activity (~5 min) • Break up into groups of 3 students representing – Policy Maker – Manufacturer – Consumer • Discuss what you would want to see in a device approval process from each perspective • Report back
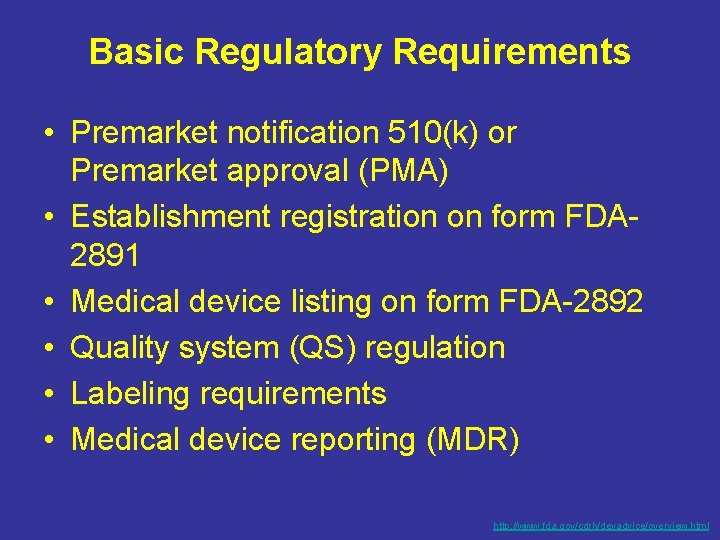
Basic Regulatory Requirements • Premarket notification 510(k) or Premarket approval (PMA) • Establishment registration on form FDA 2891 • Medical device listing on form FDA-2892 • Quality system (QS) regulation • Labeling requirements • Medical device reporting (MDR) http: //www. fda. gov/cdrh/devadvice/overview. html

Device Approval Process • Device + intended use considered together • Manufacturer submits request for marketing approval • Advisory panel – One consumer representative (non-voting) – One industry representative (non-voting) – Physicians and scientists • FDA not required to follow recommendations of panel, although they usually do

Steps in Device Approval Process • Device, drug, biologic, combo? • If device, Class I, II, or III? • 510(k) or PMA pathway? • If PMA passes, get IDE • 2 phases of clinical trials • If efficacy shown, submit pre-market notification • Post-market surveillance
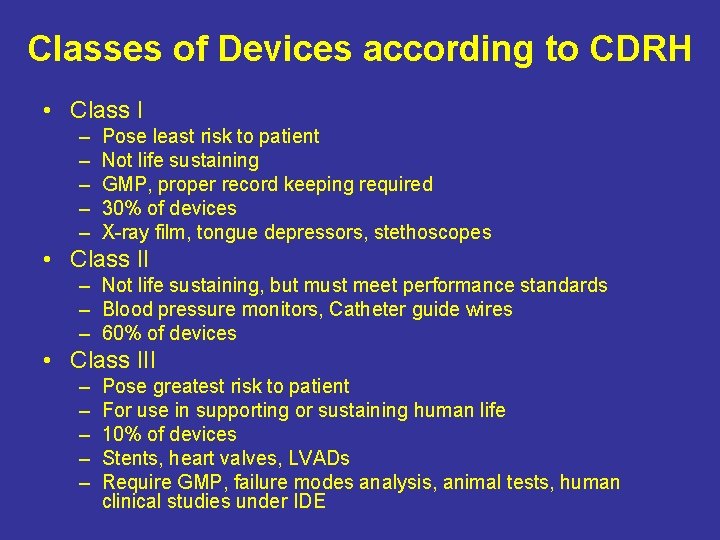
Classes of Devices according to CDRH • Class I – – – Pose least risk to patient Not life sustaining GMP, proper record keeping required 30% of devices X-ray film, tongue depressors, stethoscopes • Class II – Not life sustaining, but must meet performance standards – Blood pressure monitors, Catheter guide wires – 60% of devices • Class III – – – Pose greatest risk to patient For use in supporting or sustaining human life 10% of devices Stents, heart valves, LVADs Require GMP, failure modes analysis, animal tests, human clinical studies under IDE

510(k) vs PMA • Substantially equivalent to a legally • Most Class III required marketed product – Before May 28 th, 1976 • Requires submission of clinical – Determined by FDA data • Most Class I exempt, most Class II • Actual approval process of device required by FDA • For description of device classification and database access, visit http: //www. fda. gov/cdrh/devadvice/ 313. html • 3 rd Party review process now available
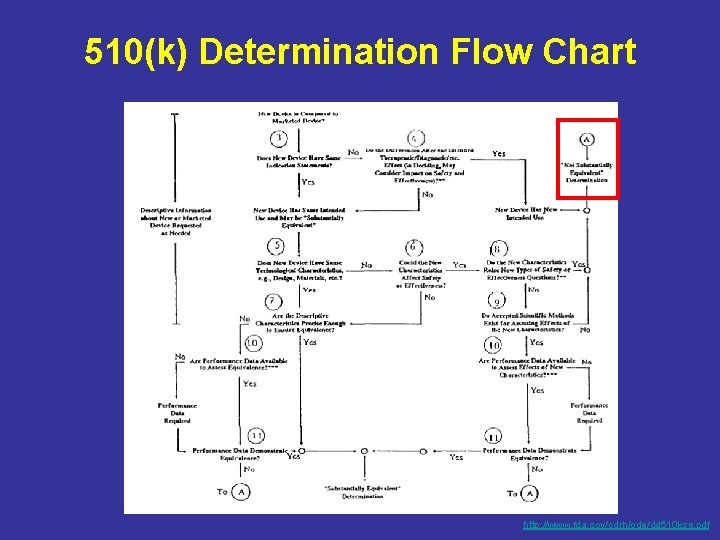
510(k) Determination Flow Chart http: //www. fda. gov/cdrh/ode/dd 510 kse. pdf

Premarket Approval (PMA) • • Device and company name Indications Device description and schematics Description of alternate practices Contraindications Potential adverse affects Summary of all pre-clinical studies
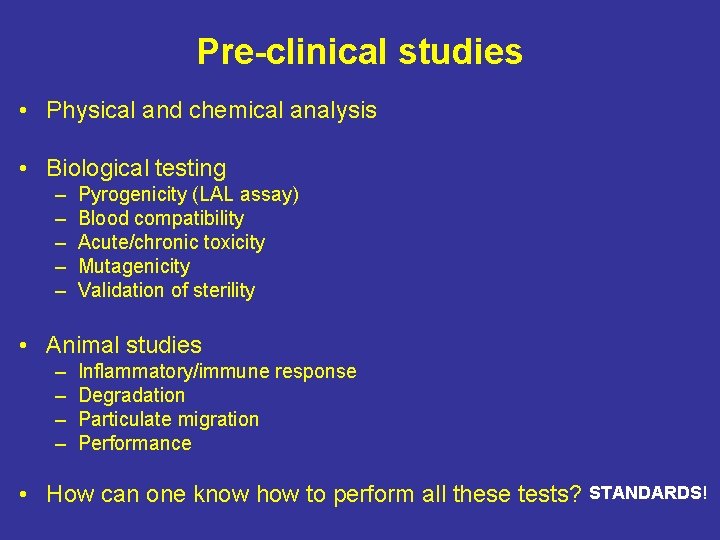
Pre-clinical studies • Physical and chemical analysis • Biological testing – – – Pyrogenicity (LAL assay) Blood compatibility Acute/chronic toxicity Mutagenicity Validation of sterility • Animal studies – – Inflammatory/immune response Degradation Particulate migration Performance • How can one know how to perform all these tests? STANDARDS!

Standards • Tripartite Agreement on Biocompatibility: FDA guidelines regarding techniques for pre-clinical testing • ASTM Committee F-04 on Medical and Surgical Materials and Devices • Standards for – Test methods: specimen prep, conditions, number of samples, data analysis – Materials: chemical and physical properties – Devices: schematics, dimensions, tolerances – Procedures: how to do something besides a test, i. e. sterilization, • FDA accepts standards such that one can write, “I did X according to Y standard” • Shift in perspective towards worldwide harmonized system – ISO 10993 document – At least 16 parts describing globally recognized standards for • • Biocompatibility screens Evaluation for ethylene oxide residues Toxicity studies (i. e. , cyto, systemic, & kinetics) Many more

IDE and Clinical Trials • Investigational Device Exemption – Enables experimental use of high risk device – Must have positive engineering and animal data • Clinical Trials – First give approval for feasibility studies with small number of patients – Then proceed to multi-center trials – Larger data sets frequently show results from small sample sets are not true

Humanitarian Use Exemption • Device designed to treat or diagnose condition that affects <4, 000 patients/year • Device would not otherwise be available without exemption • No comparable device is available • Patients will not be exposed to unreasonable or significant risk of injury or illness by device

Medical Device Reporting • System to detect device related problems in a timely manner • Serious injuries or deaths that may have been caused by or related to a a medical device must be reported to the manufacturer of the device within 10 days • Must be reported to the FDA within 10 days

Steps in Device Approval Process • Device, drug, biologic, combo? • If device, Class I, II, or III? • 510(k) or PMA pathway? • If PMA passes, get IDE • 2 phases of clinical trials • If efficacy shown, submit pre-market notification • Post-market surveillance

Recently Approved Devices • http: //www. accessdata. fda. gov/scripts/cdrh /cfdocs/cf. Topic/MDA/mda-list. cfm? list=1

Device Development Costs $$$ • Who funds development? • What is the typical government grant process?

R&D Funding for Biomedical Research • Federal government – Funds ~ 35% of all medical research in US • Mostly funded through NIH – Current NIH budget: ~$28 billion/year – NIH budget doubled from 1998 -2003 – Last year 0% increase – Focus is on basic research
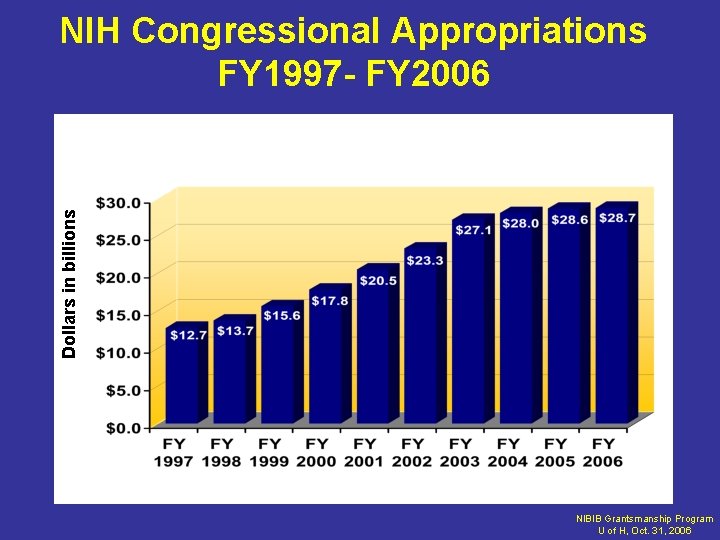
Dollars in billions NIH Congressional Appropriations FY 1997 - FY 2006 NIBIB Grantsmanship Program U of H, Oct. 31, 2006
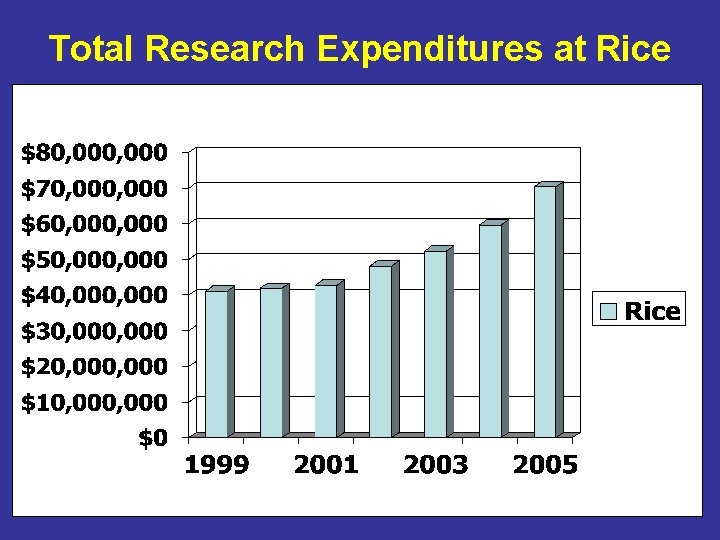
Total Research Expenditures at Rice
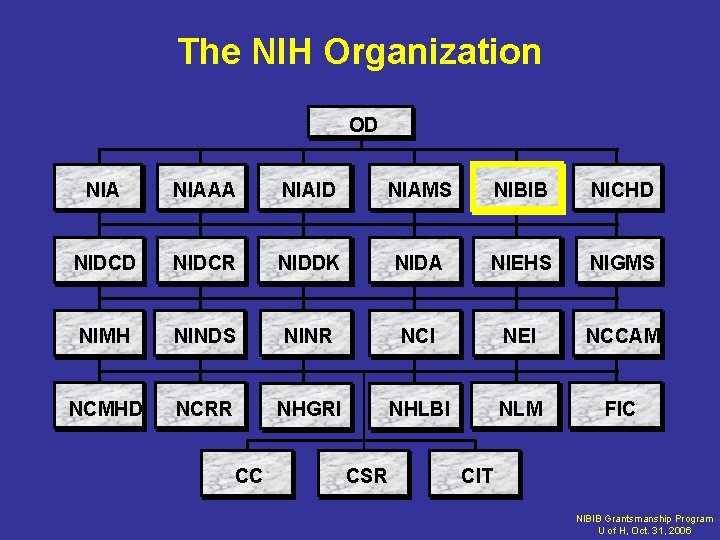
The NIH Organization OD NIAAA NIAID NIAMS NIBIB NICHD NIDCR NIDDK NIDA NIEHS NIGMS NIMH NINDS NINR NCI NEI NCCAM NCMHD NCRR NHGRI NHLBI NLM FIC CC CSR CIT NIBIB Grantsmanship Program U of H, Oct. 31, 2006

NIH • • • • • National Cancer Institute National Eye Institute National Heart, Lung, and Blood Institute National Human Genome Research Institute National Institute on Aging National Institute on Alcohol Abuse and Alcoholism National Institute of Allergy and Infectious Diseases National Institute of Arthritis and Musculoskeletal and Skin Diseases National Institute of Biomedical Imaging and Bioengineering National Institute of Child Health and Human Development National Institute on Deafness and Other Communication Disorders National Institute of Dental and Craniofacial Research National Institute of Diabetes and Digestive and Kidney Diseases National Institute on Drug Abuse National Institute of Environmental Health Sciences National Institute of General Medical Sciences National Institute of Neurological Disorders and Stroke National Institute of Nursing Research National Library of Medicine

The NIBIB Vision To profoundly change health care by pushing the frontiers of technology to make the possible a reality. NIBIB Grantsmanship Program U of H, Oct. 31, 2006

A big challenge for the NIBIB is promoting multidisciplinary research • Clinicians, biologists and engineers speak in different languages • Clinicians and biologists may not know what is technically possible; engineers may not know the biomedical problems • Continued, ongoing collaboration essential NIBIB Grantsmanship Program U of H, Oct. 31, 2006

Current NIBIB Grant Portfolio Areas • • • • Biosensors Biomaterials Biomechanics Biomedical Informatics Computational Biology Drug & Gene Delivery Systems Lab-on-a-Chip Devices/Microsystems Medical Devices & Implant Science Nanotechnology Rehabilitation Engineering Surgical Tools & Techniques Telemedicine Tissue Engineering • Imaging Agents & Molecular Probes • Image Displays • Image Guided Therapies & Interventions • Image Perception • Image Processing • Magnetic, Biomagnetic & Bioelectric Devices • Magnetic Resonance Imaging & Spectroscopy • Nuclear Medicine • Optical Imaging & Spectroscopy • Ultrasound and Acoustics • X ray, Electron & Ion Beam NIBIB Grantsmanship Program U of H, Oct. 31, 2006
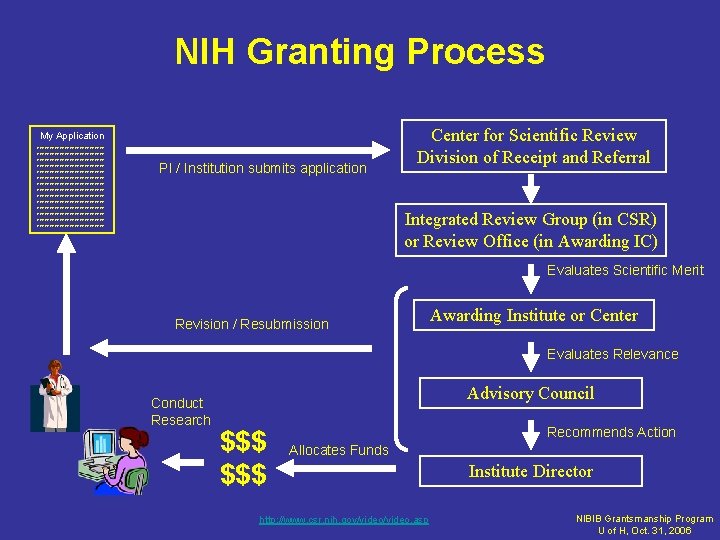
NIH Granting Process My Application xxxxxxxxxxxxxxxxxxxxxxxxxxx xxxxxxxxxxxxxxxxxxxxxxxxxxx xxxxxxxxxxxxxxxxxxxxxxxxxxx xxxxxxxxxxxxxxxxxxxxxxxxxxx PI / Institution submits application Center for Scientific Review Division of Receipt and Referral Integrated Review Group (in CSR) or Review Office (in Awarding IC) Evaluates Scientific Merit Revision / Resubmission Awarding Institute or Center Evaluates Relevance Conduct Research Advisory Council $$$ Recommends Action Allocates Funds http: //www. csr. nih. gov/video. asp Institute Director NIBIB Grantsmanship Program U of H, Oct. 31, 2006

Scores and Funding Level • ~1/3 go unscored (triaged) • ~2/3 are scored – 100 best score – 500 worst score – Typically need score of 100 -170 to be funded – Approximately 10 -15% of submitted proposals are funded

For More Information, See the Following Sources • http: //www. fda. gov/cdrh/devadvice/overview. html • Biomaterials Science: An Introduction to Materials in Medicine, 2 nd Ed. Chap 10. Elsevier Academic Press. Editors: Ratner, Hoffman, Schoen, Lemons. • Materials from NIBIB Grantsmanship Program, University of Houston, Oct. 31, 2006

Index Cards • What was the muddiest point of this lecture? • What was the clearest point in this lecture? HHMI survey, see you on Thursday!
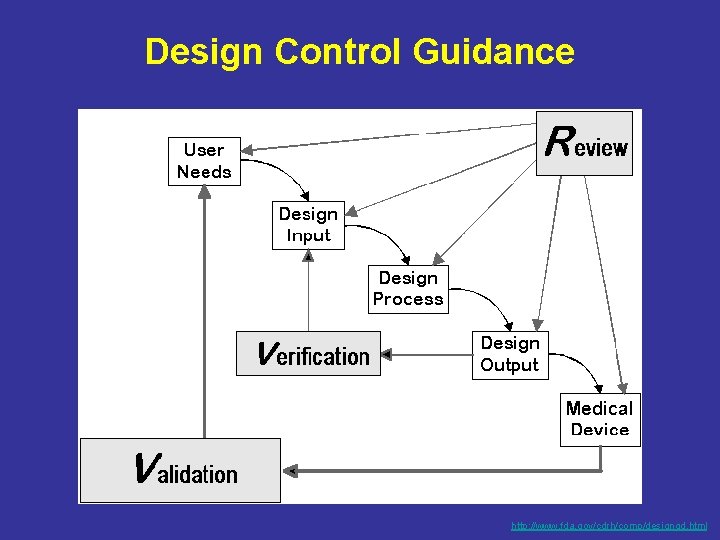
Design Control Guidance http: //www. fda. gov/cdrh/comp/designgd. html

Types of Universities • Carnegie Classification – Taxonomy of colleges and universities • Doctorate-Granting Institutions – Research Universities /Very High Research Activity – Research Universities/ High Research Activity – Doctoral/Research Universities • Master’s Colleges & Universities • Baccalaureate Colleges – http: //www. carnegieclassificationpreview. org/index. aspx Rice
- Slides: 40