Biodistribution of monoclonal antibody aggregates upon SC administration

Biodistribution of monoclonal antibody aggregates upon SC administration Grzegorz Kijanka Division of Drug Delivery Technology Leiden Academic Centre for Drug Research (LACDR) 24 th February 2015

Table of contents. 1. Introduction 2. Aim of the project 3. Experimental setup 4. Results 5. Conclusions 6. Acknowledgements
1. Protein aggregates - immunogenicity Immunogenicity Product related factors: - Origin - Sequence - PTMs - Formulation - Impurities (aggregates) aggregates -. . . Patient related factors: - Age - Genetic background - Disease - Immunological state -. . . Therapy related factors: - Dose - Duration - Aplication route - Co-treatment -. . .
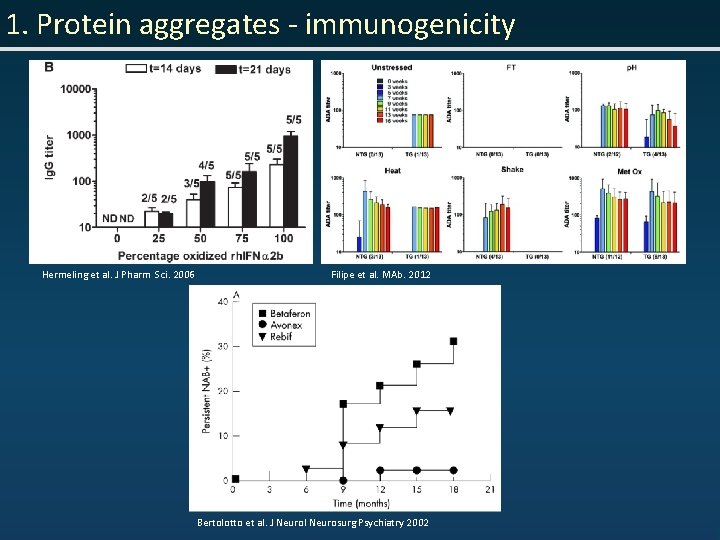
1. Protein aggregates - immunogenicity Hermeling et al. J Pharm Sci. 2006 Filipe et al. MAb. 2012 Bertolotto et al. J Neurol Neurosurg Psychiatry 2002

1. Protein aggregates • “Protein aggregate”– assembly of protein molecules with higher MW than desired species • Protein aggregates characterization: - size - morphology - secondary/tertiary structure - reversibility - covalent modifications -. . .
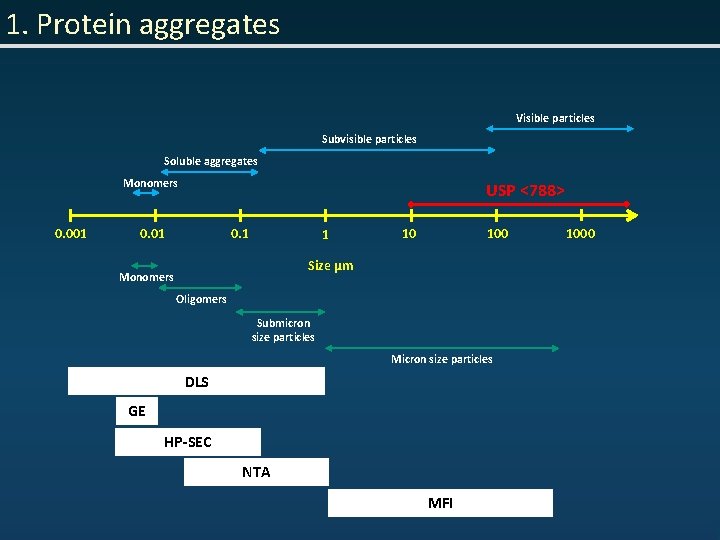
1. Protein aggregates Visible particles Subvisible particles Soluble aggregates Monomers 0. 001 USP <788> 0. 01 0. 1 1 10 100 Size µm Monomers Oligomers Submicron size particles Micron size particles DLS GE HP-SEC NTA MFI 1000
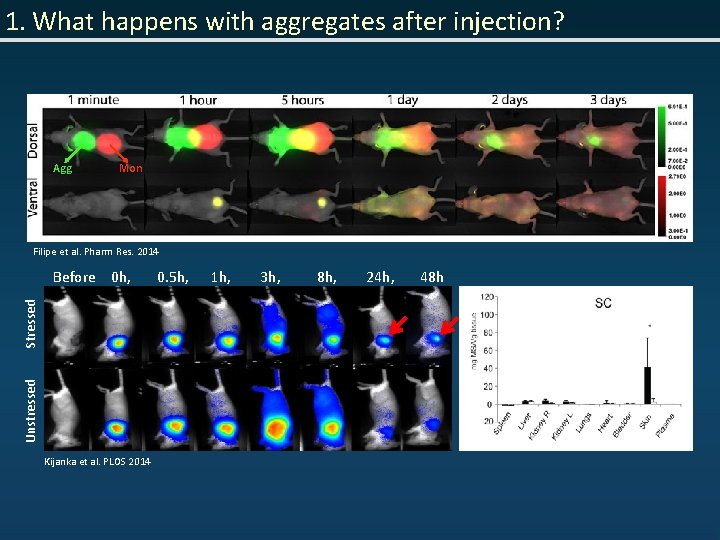
1. What happens with aggregates after injection? Agg Mon Filipe et al. Pharm Res. 2014 Unstressed Stressed Before 0 h, Kijanka et al. PLOS 2014 0. 5 h, 1 h, 3 h, 8 h, 24 h, 48 h

1. What happens with aggregates after injection? • Questions: – Which aggregates (dimers, oligomers, sub and/or micron size particles) contribute the most to altered disposition from injection spot? – How do different aggregates influence the biodistribution of protein? – Does the origin of protein (self/foreign) influence the biodistribution of aggregates? – Can (altered) biodistribution of aggegated protein increase the risk of immunogenicity? – Is it possible, by measuring the biodistribution, to select the most immunogenic size range of protein aggregates?

2. Aim of the project. • To determine the biodistribution of different Ig. G’s size species upon SC injection
3. Key materials • Model proteins: – rh. Ig. G 1 (r 347) – rm. Ig. G 1 (1 A 7) • Animal model: SKH 1 mice – Hairless strain – Immunecompetent • Fluorescent dye: IR Dye 800 CW – Fluorescence in near infra-red, good penetration through tissues – Very stable in vivo
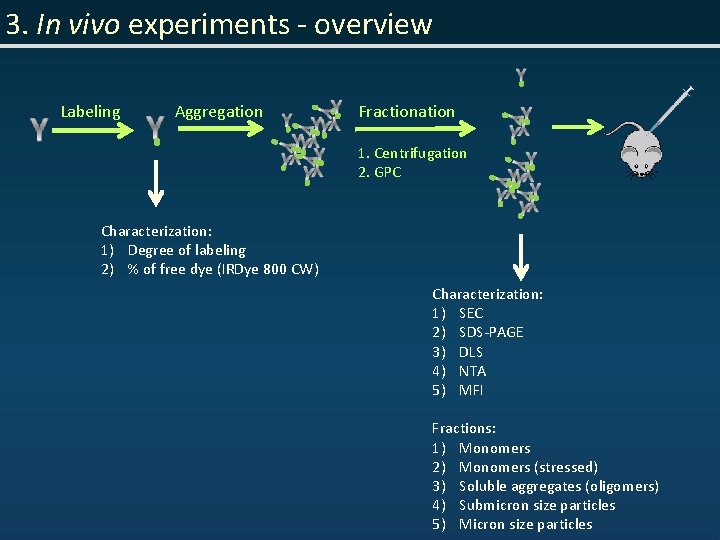
3. In vivo experiments - overview Labeling Aggregation Fractionation 1. Centrifugation 2. GPC Characterization: 1) Degree of labeling 2) % of free dye (IRDye 800 CW) Characterization: 1) SEC 2) SDS-PAGE 3) DLS 4) NTA 5) MFI Fractions: 1) Monomers 2) Monomers (stressed) 3) Soluble aggregates (oligomers) 4) Submicron size particles 5) Micron size particles

3. In vivo experiments - overview Imaging Euthanasia 1) SC injection 2) 50 µg of Ig. G (in 100 µl PBS) 3) 1 A 7 and r 347 1) 1 hr, 24 hrs, 7 days Collecting organs / tissues 1) Tissues: blood, urine, muscle, skin (hind leg), skin (injection spot) 2) Organs: thymus, lung, heart, liver, kidney, spleen, (lymph nodes) Biodistribution 1) Ex vivo organs imaging 2) Quantitative biodistribution
3. Aggregation and fractionation • Final aggregation conditions – r 347 -IR Dye 800 CW conjugates 1 mg/ml, p. H=4. 6, 63˚C, 1 hr + 30 min of stirring (700 rpm) – 1 A 7 -IR Dye. CW conjugates 1 mg/ml, p. H=4. 6, 55˚C, 1 hr + 30 min of stirring (700 rpm) • Fractionation via centrifugation (3000 g, 10 min, RT) – “Pellet”: fraction enriched with micron size particles – “Supernatant”: submicron size particles • Fractionation via GPC – Monomers subjected to stress conditions: “GPC Monomers ” – Oligomers: “GPC Oligomers”
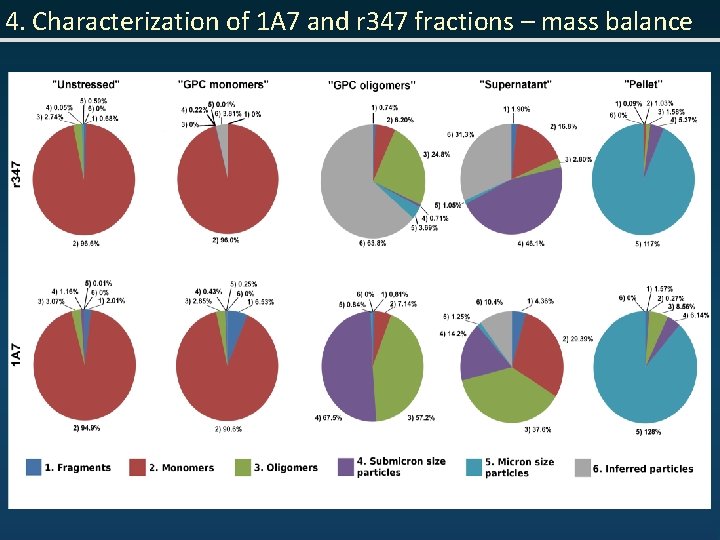
4. Characterization of 1 A 7 and r 347 fractions – mass balance
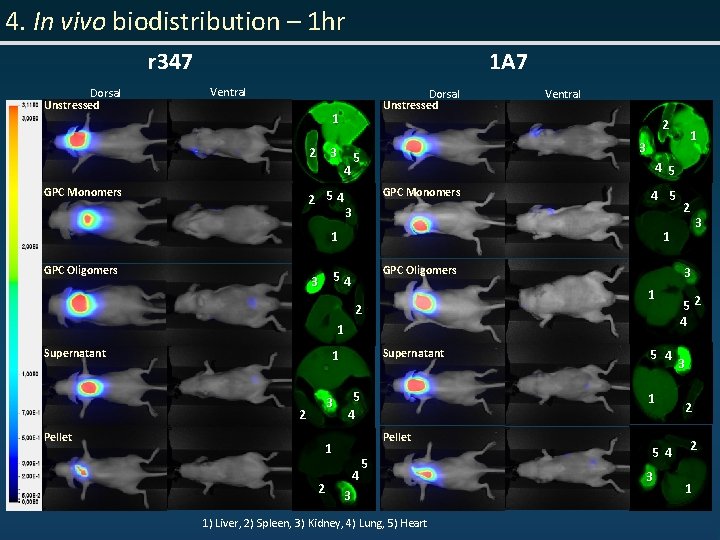
4. In vivo biodistribution – 1 hr r 347 Dorsal Unstressed 1 A 7 Ventral Dorsal Unstressed 1 2 3 GPC Monomers Ventral 2 4 2 54 5 GPC Monomers 3 4 5 1 GPC Oligomers 1 Supernatant 1 3 2 Pellet 5 4 2 4 5 4 1 Pellet 1 5 3 1) Liver, 2) Spleen, 3) Kidney, 4) Lung, 5) Heart 3 3 1 2 Supernatant 2 1 54 3 1 3 5 4 3 52 4 3 2 2 1
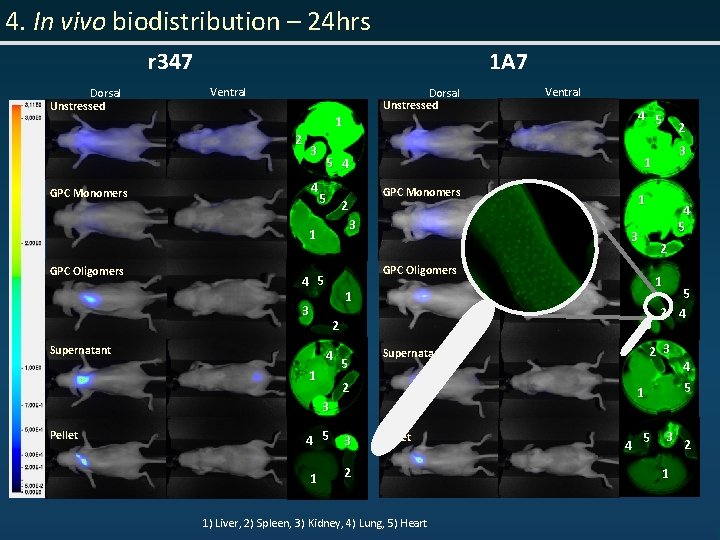
4. In vivo biodistribution – 24 hrs r 347 Dorsal Unstressed 1 A 7 Ventral Dorsal Unstressed 2 3 4 GPC Monomers 1 4 5 5 4 1 5 GPC Monomers 2 3 1 GPC Oligomers 3 3 1 5 1 3 4 5 3 1 2 Pellet 1) Liver, 2) Spleen, 3) Kidney, 4) Lung, 5) Heart 5 2 4 22 33 Supernatant 2 4 5 2 1 4 3 1 2 Supernatant 2 1 GPC Oligomers 4 5 3 Pellet Ventral 1 5 44 55 33 2 11
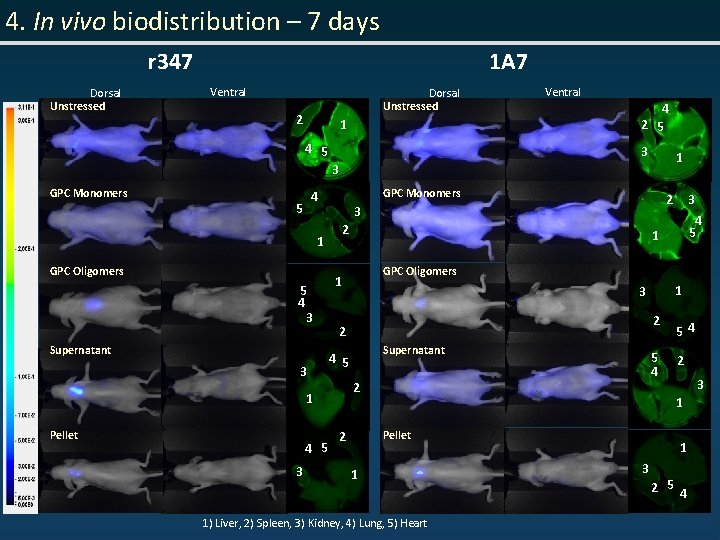
4. In vivo biodistribution – 7 days r 347 Dorsal Unstressed 1 A 7 Ventral Dorsal Unstressed 2 1 4 5 Ventral 4 2 5 3 1 3 GPC Monomers 4 5 1 GPC Oligomers 5 4 3 Supernatant 3 2 4 5 3 3 3 4 5 1 GPC Oligomers 1 1 3 2 Supernatant 4 5 2 1 Pellet 2 54 5 4 2 3 1 Pellet 2 2 1 1) Liver, 2) Spleen, 3) Kidney, 4) Lung, 5) Heart 1 3 2 5 4
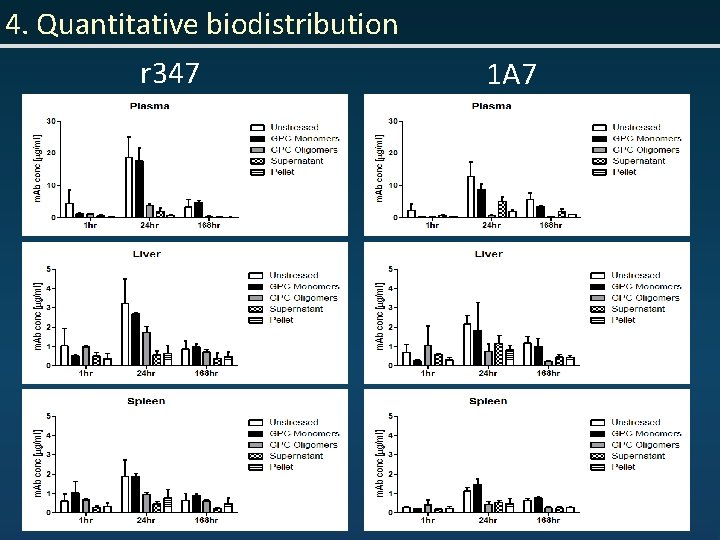
4. Quantitative biodistribution r 347 1 A 7
5. Conclusions • Similar biodistribution of murine (1 A 7) and human (r 347) antibody upon SC injection • Monomeric antibodies, even subjected to stress conditions, nicely distribute within the whole body of animals • Presence of aggregates (both sub micron size and micron size) alters biodistribution • There is no specific tissue/organ in which aggregated antibodies accumulate (measurably) • Fluorescent „dots” were detected in spleens and lymph nodes of some animals injected with „ 1 A 7 Oligomers”

6. Acknowladgements • LACDR – Wim Jiscoot – Stefan Romerijn – Eleni Varypataki • Med Immune – Jared Bee – Xu Liu – Kirsten Schneider-Ohrum – Robert Kubiak – Melissa Coughlin – Steven Bishop – Richard Remmele – Mark Schenerman – Srilatha Kuntumalla – Maria Andrea Miller – Norman Peterson – Wendy White • LUMC – Clemens Löwik – Ivo Que

Thank you for your attention!
Imaging control 1 4 23 5
- Slides: 22