Biochemistry Water Properties of water Neutral compound but

Biochemistry

Water • Properties of water: – Neutral compound, but. . – Polar: water molecules are attracted to other water molecules due to opposite charges • Ex: Water droplets – Cohesion: Same substances are attracted • Water is attracted to water – Adhesion: Different substances are attracted (water is attracted to something else) • Ex: Water droplets on your car windshield
Biochemistry • Carbon compounds= Organic compounds – Large molecules (polymers) that are formed by small molecules (monomer) – Water is removed to join monomers to make polymersdehydration synthesis • De-without • hydra=water synthesis= to make 4 groups of Organic Compounds: 1. 2. 3. 4. Carbohydrates Lipids Proteins Nucleic Acids
Carbohydrates • Contain Carbon, Hydrogen, and Oxygen in a 1: 2: 1 ratio • Main source of energy in the body • Immediate energy-breakdown sugars. – Sugar that is stored is starch (complex carbohydrate) • Three types of carbohydrates: 1. Monosaccharide: Simple sugar 2. Disaccharide: Double sugar 3. Polysaccharide: Many sugars
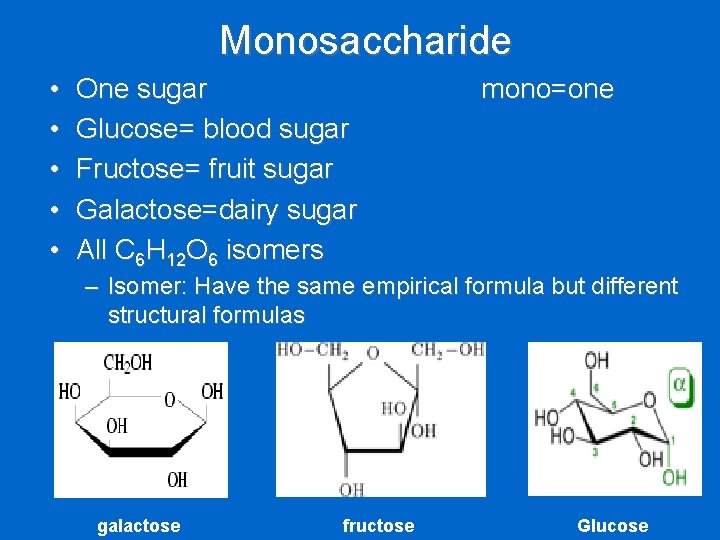
Monosaccharide • • • One sugar Glucose= blood sugar Fructose= fruit sugar Galactose=dairy sugar All C 6 H 12 O 6 isomers mono=one – Isomer: Have the same empirical formula but different structural formulas galactose fructose Glucose
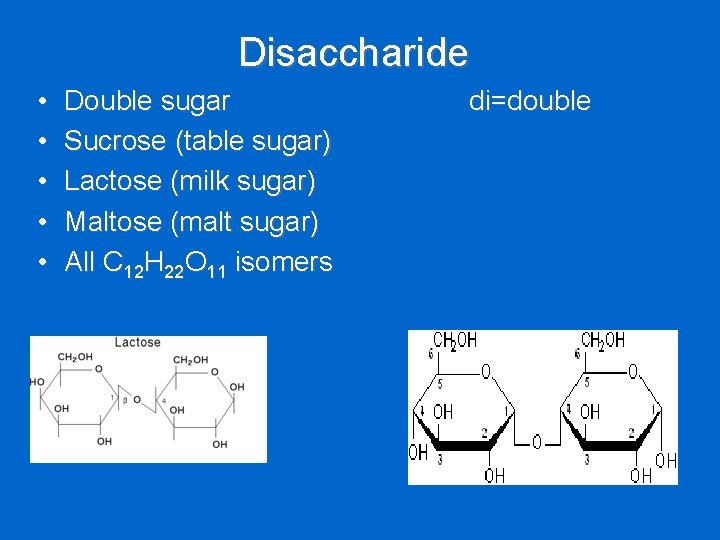
Disaccharide • • • Double sugar Sucrose (table sugar) Lactose (milk sugar) Maltose (malt sugar) All C 12 H 22 O 11 isomers di=double
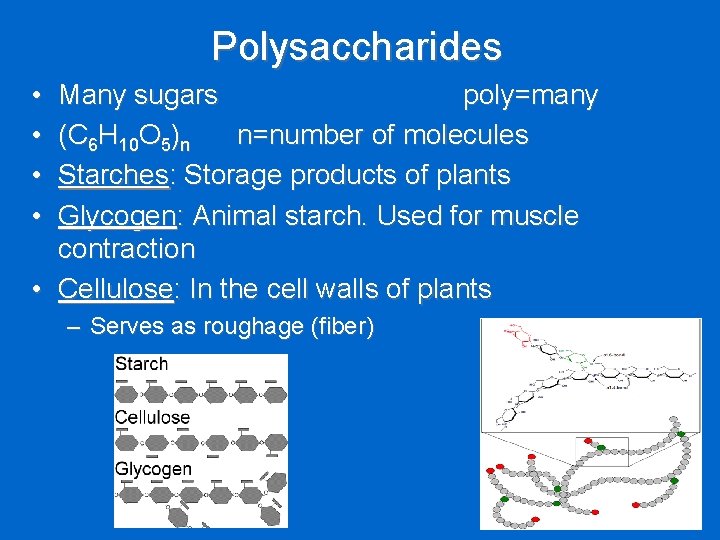
Polysaccharides • • Many sugars poly=many (C 6 H 10 O 5)n n=number of molecules Starches: Storage products of plants Glycogen: Animal starch. Used for muscle contraction • Cellulose: In the cell walls of plants – Serves as roughage (fiber)
Lipids • Contain C, H, O in a greater than 1: 2: 1 ratio • Lots of energy stored here! • Stores energy efficiently (large number of C to H bonds) • Make up biological membranes, and waterproof coverings. • Are hydrophobic (scared of water) • Common lipids include fats, oils, waxes, and steroids
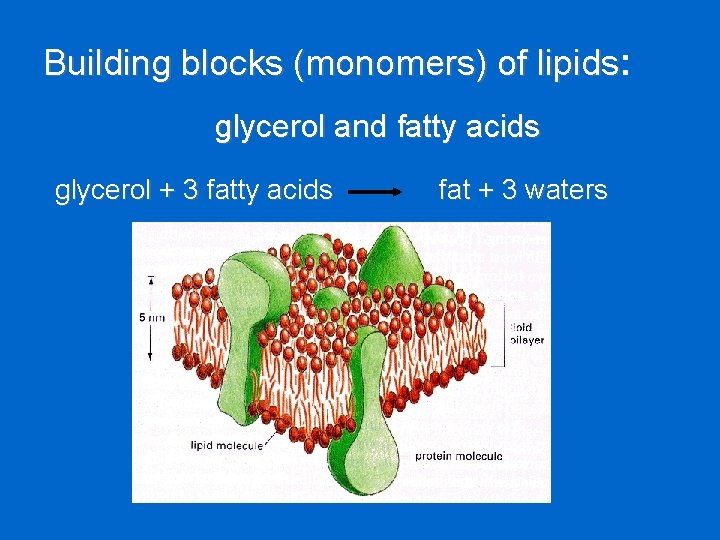
Building blocks (monomers) of lipids: lipids glycerol and fatty acids glycerol + 3 fatty acids fat + 3 waters
Proteins • Contain C, H, O, N • Control rates of reactions, regulate cell processes • Building blocks (monomers) – amino acids -20 different amino acids
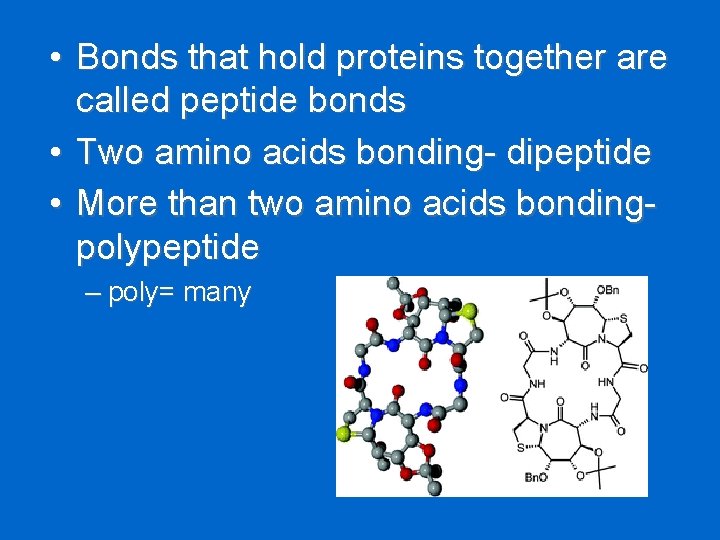
• Bonds that hold proteins together are called peptide bonds • Two amino acids bonding- dipeptide • More than two amino acids bondingpolypeptide – poly= many
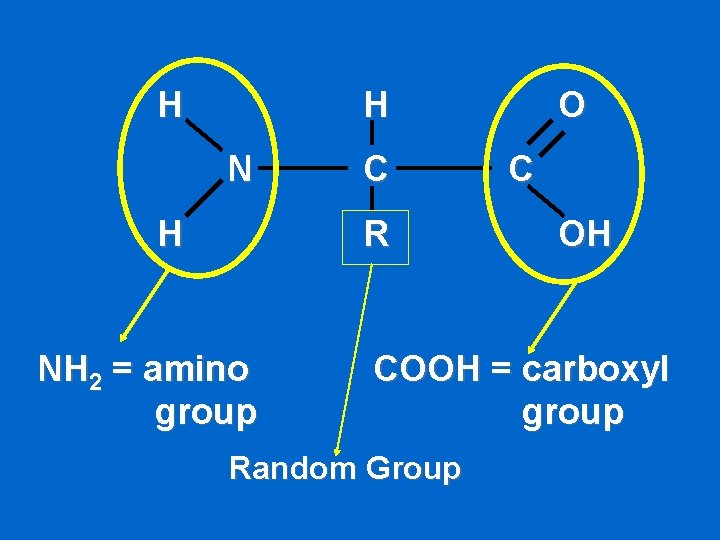
H H N H C R NH 2 = amino group O C OH COOH = carboxyl group Random Group
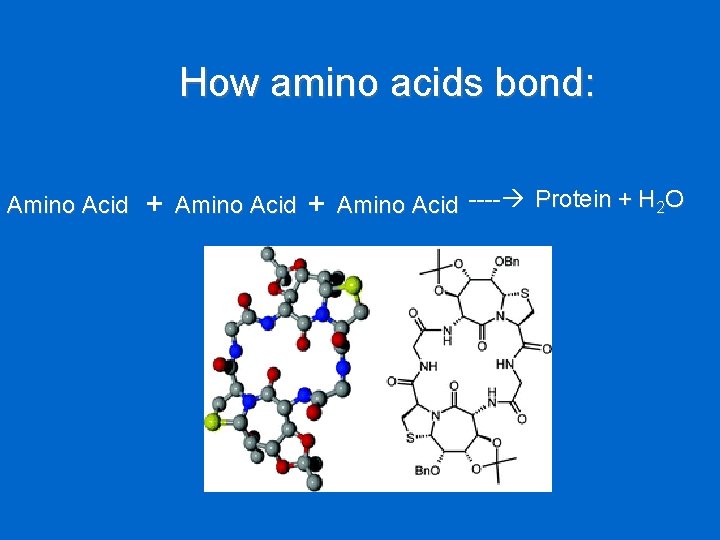
How amino acids bond: Amino Acid + Amino Acid ---- Protein + H 2 O
Enzymes • Proteins that act as catalysts to speed up or slow down chemical reactions that take place in cells • Lower activation energy -The amount of energy needed to start the reaction • Very specific – may act on only one kind of chemical reaction (Lock and Key Hypothesis)
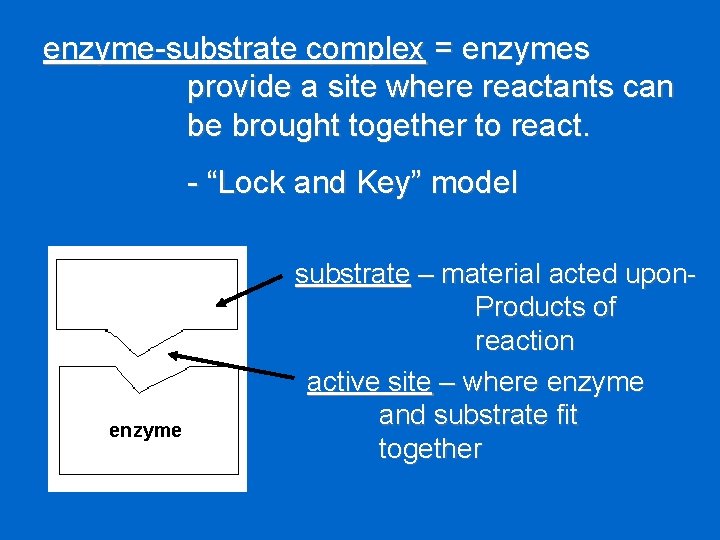
enzyme-substrate complex = enzymes provide a site where reactants can be brought together to react. - “Lock and Key” model enzyme substrate – material acted upon. Products of reaction active site – where enzyme and substrate fit together
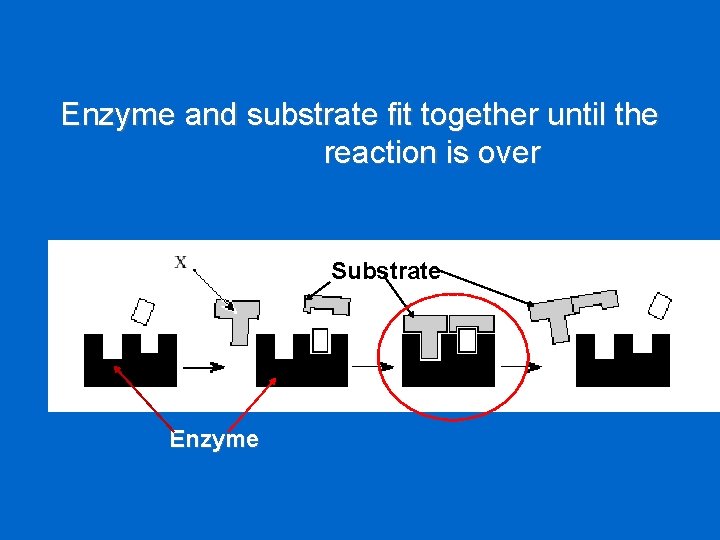
Enzyme and substrate fit together until the reaction is over Substrate Enzyme
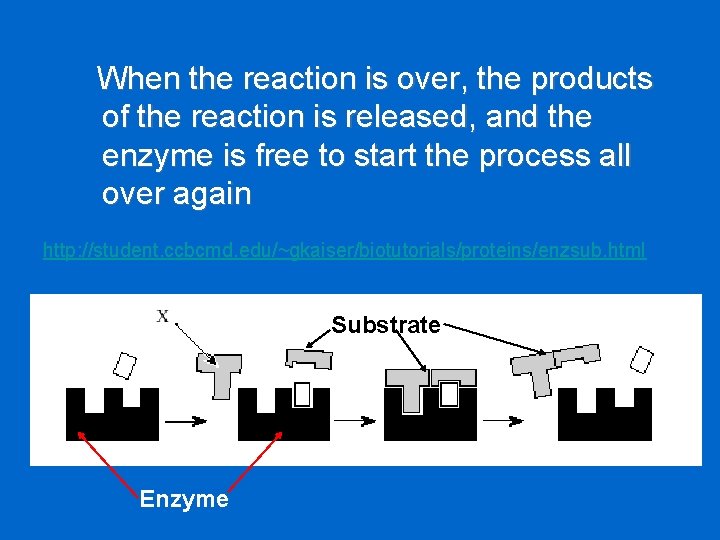
When the reaction is over, the products of the reaction is released, and the enzyme is free to start the process all over again http: //student. ccbcmd. edu/~gkaiser/biotutorials/proteins/enzsub. html Substrate Enzyme
Nucleic Acids • Contain C, H, O, N, P • Controls the synthesis (creation) of proteins • Store and transmit genetic information • Monomers are nucleotides
2 kinds of nucleic acids 1. DNA – deoxyribonucleic acid • Controls genetic traits 2. RNA – ribonucleic acid • Helps make proteins
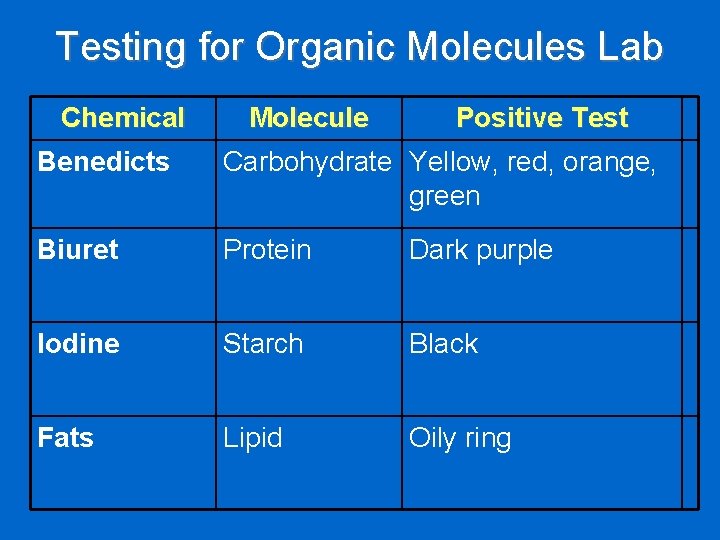
Testing for Organic Molecules Lab Chemical Molecule Positive Test Benedicts Carbohydrate Yellow, red, orange, green Biuret Protein Dark purple Iodine Starch Black Fats Lipid Oily ring
- Slides: 20