Biochemistry Water and p H Water An important

Biochemistry Water and p. H
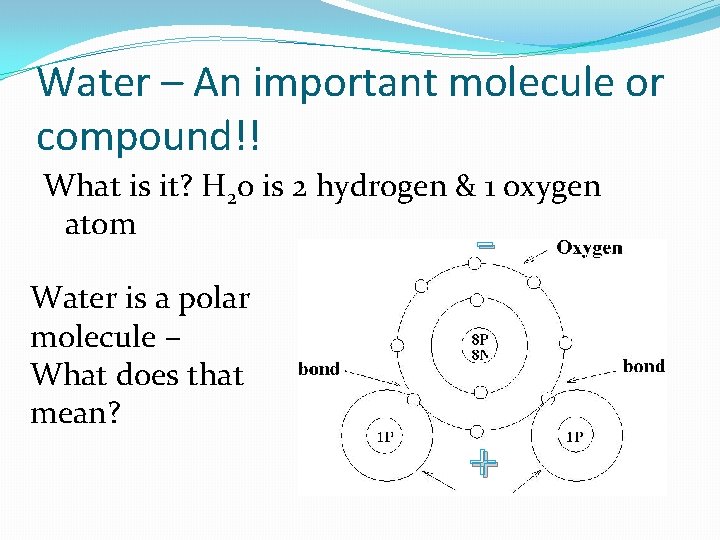
Water – An important molecule or compound!! What is it? H 20 is 2 hydrogen & 1 oxygen atom - Water is a polar molecule – What does that mean? +

Bonding Water Together �Water is a polar molecule that is attracted to other polar molecules…not non-polar Can you think of an example of something that might be non-polar? �Water molecules form a weak bond between the Hydrogen (H) atom and a negatively charged region of another molecule of water.

Polar vs. Non-Polar �Hydrophilic – substances that have an affinity for water Does not mean that the substance has to dissolve – cotton fibers have a high affinity for water, so make great towels!!! �Hydrophobic – substances that fear or repel water (“water fearing”)-form clusters called hydrophobic interactions. Ex. Vegetable oil
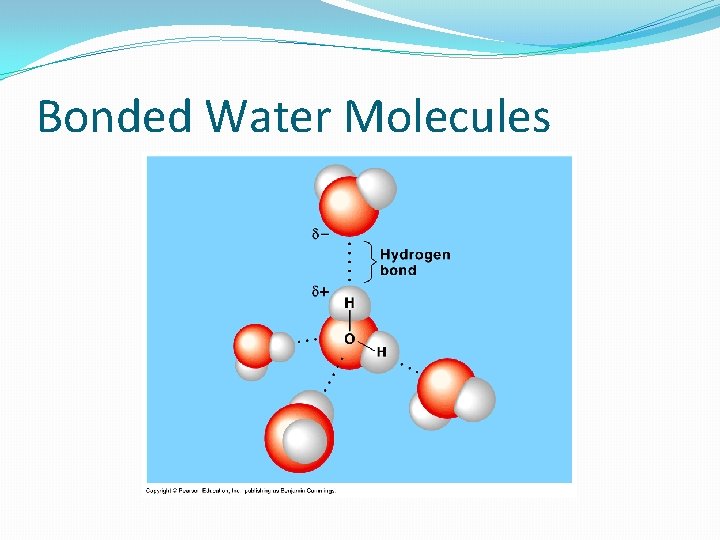
Bonded Water Molecules
Properties of Water �Cohesion – Water molecules are attracted to each other, leaves a sphere shape �Adhesion – Water molecules are attracted to other molecules �Capillary Action – Water molecules move up the plant, from the roots to the leaves, using both adhesion and cohesion.

Capillary Action – Zips up the Xylem

Properties of Water �Surface Tension – Cohesion keeps water molecules together and forms a thin layer on top of the water.

Mixing Water: Solutions & Suspensions 1) Solutions – Particles are evenly distributed, can not tell where one ends and the other begins. Ex. Kool Aid Solute – What is being mixed. Solvent – What is doing the mixing. Water is considered the universal solvent Can dissolve almost anything.

Mixing Water 2) Suspensions – Particles are not evenly distributed, no matter how much you mix, it will still fall out of solution. Ex. Sand Water

When you split a water molecule �End up with H+ and OH-. This leads us to p. H. �p. H is a measurement of hydrogen ions. �When there is more H+ ions it is an acid When there is more OH- ions it is a base. �The p. H Scale goes from 1 to 14, with 7 being neutral. Acids: 1 strong acid, 6 weak acid Bases: 14 strong base, 8 weak base
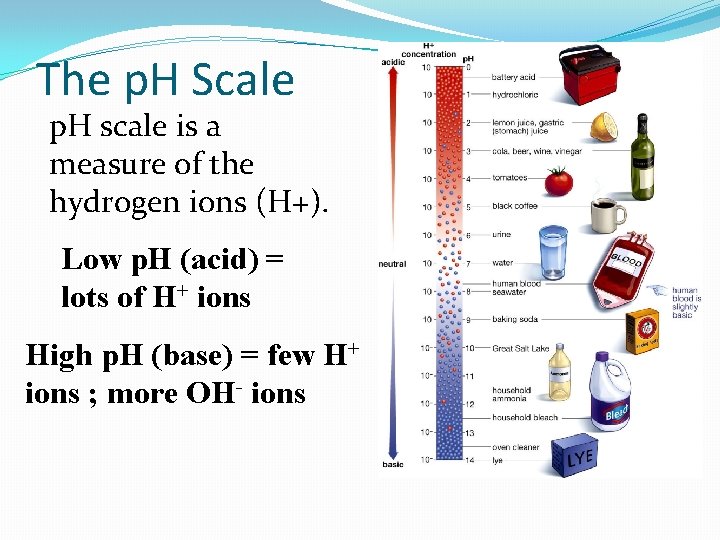
The p. H Scale p. H scale is a measure of the hydrogen ions (H+). Low p. H (acid) = lots of H+ ions High p. H (base) = few H+ ions ; more OH- ions
- Slides: 12