Biochemistry The Organic Molecules of Life Organic Compounds
Biochemistry The Organic Molecules of Life
Organic Compounds n All Organic compounds contain CARBON
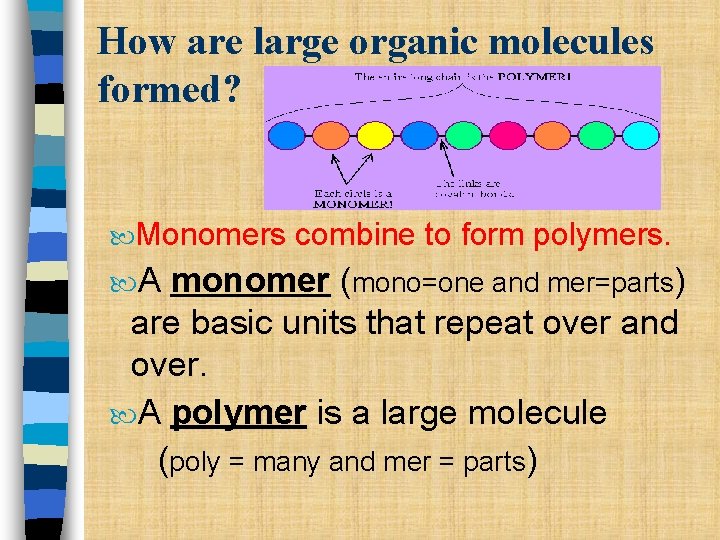
How are large organic molecules formed? Monomers A combine to form polymers. monomer (mono=one and mer=parts) are basic units that repeat over and over. A polymer is a large molecule (poly = many and mer = parts)
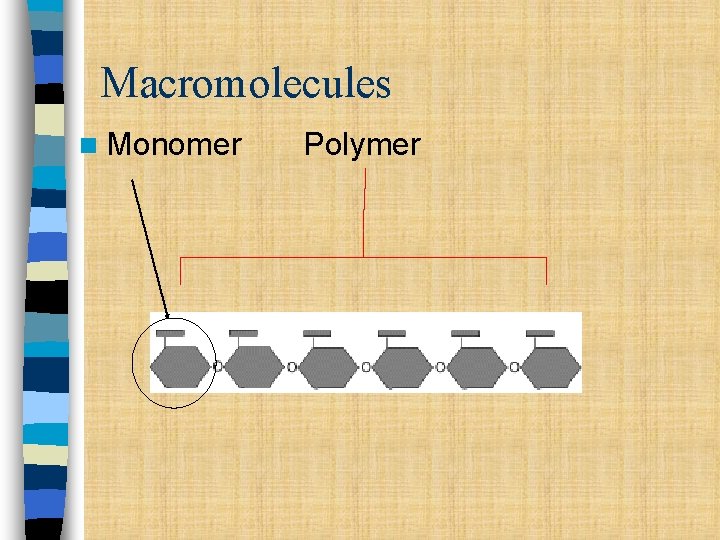
Macromolecules n Monomer Polymer
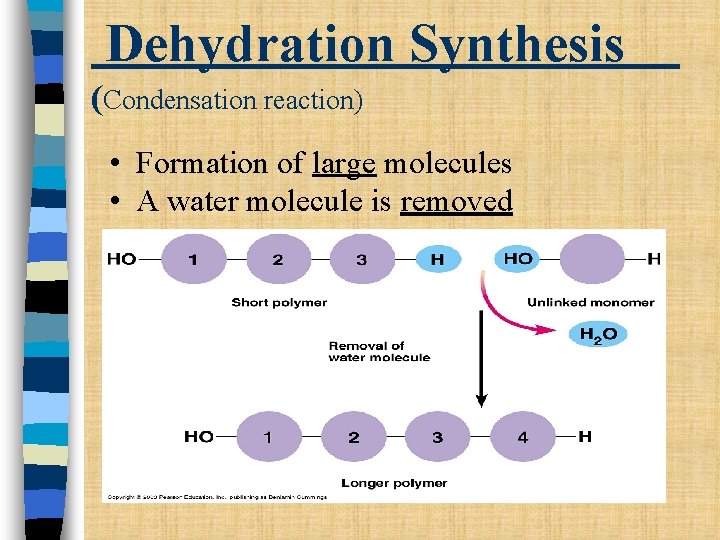
Dehydration Synthesis (Condensation reaction) • Formation of large molecules • A water molecule is removed

Hydrolysis n Hydro = water; lysis = to separate n Polymers are broken down into monomers. n Water is added and the polymer comes apart. n Hydrolysis is the reverse of dehydration synthesis
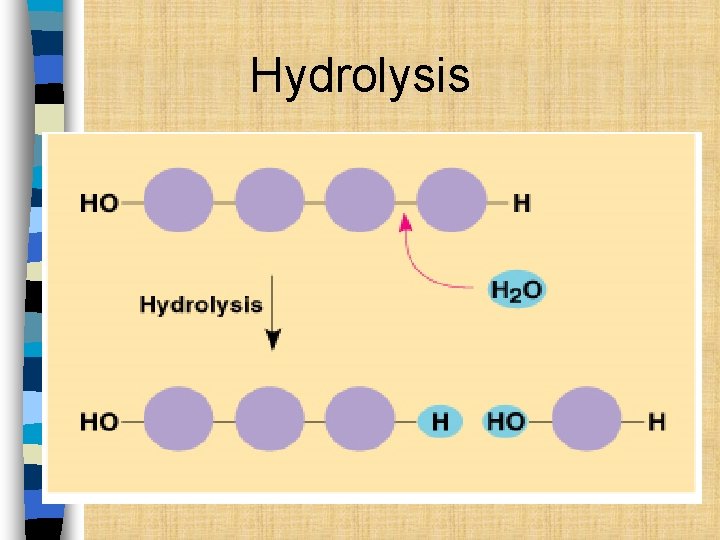
Hydrolysis

Animation- dehydration synthesis and hydrolysis n Dehydration Synthesis Disaccharide You. Tube. wmv

Carbohydrates

Testing for carbohydrates SUGAR n Benedict’s Reagent turns orange in the presence of simple sugars STARCH n Iodine turns black in the presence of starch
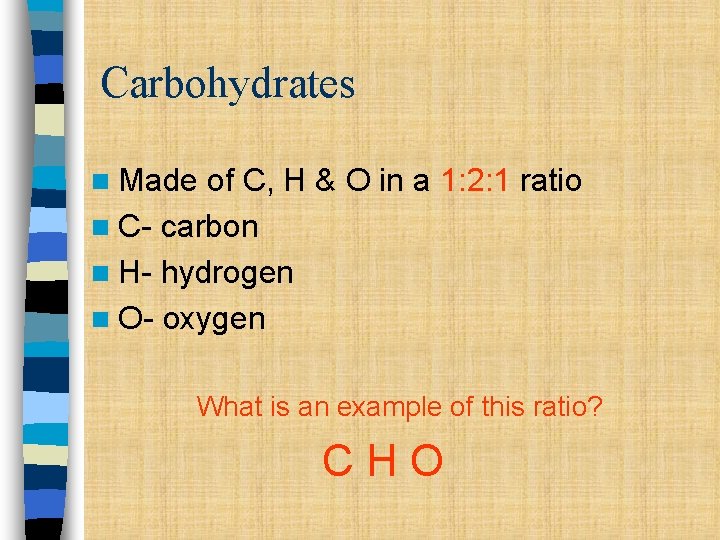
Carbohydrates n Made of C, H & O in a 1: 2: 1 ratio n C- carbon n H- hydrogen n O- oxygen What is an example of this ratio? CHO
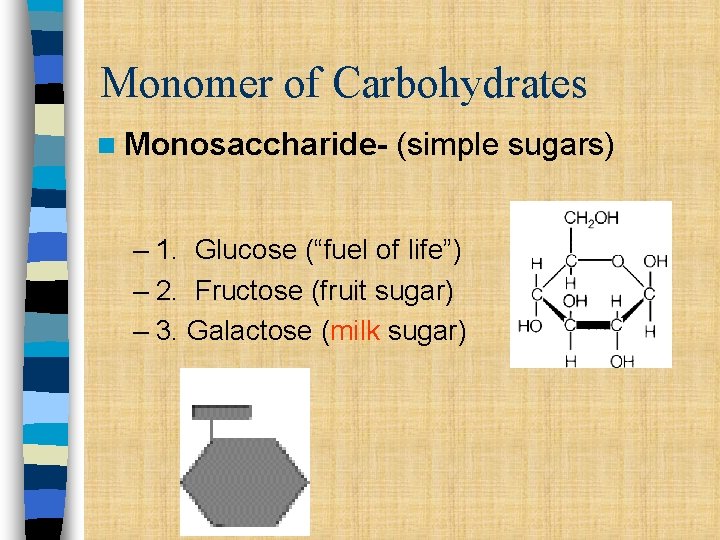
Monomer of Carbohydrates n Monosaccharide- (simple sugars) – 1. Glucose (“fuel of life”) – 2. Fructose (fruit sugar) – 3. Galactose (milk sugar)

Carbohydrates Function: – Used as a main source of ENERGY n Examples Pasta, bread, soda, Kool-Aid, candy, fruit juice
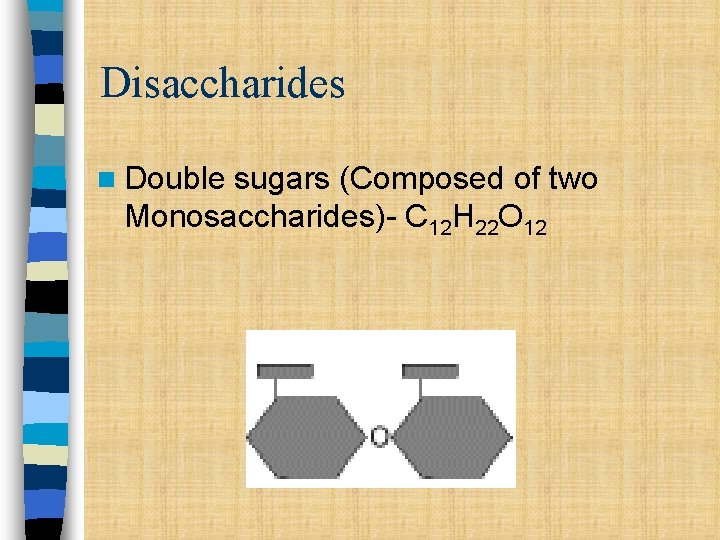
Disaccharides n Double sugars (Composed of two Monosaccharides)- C 12 H 22 O 12
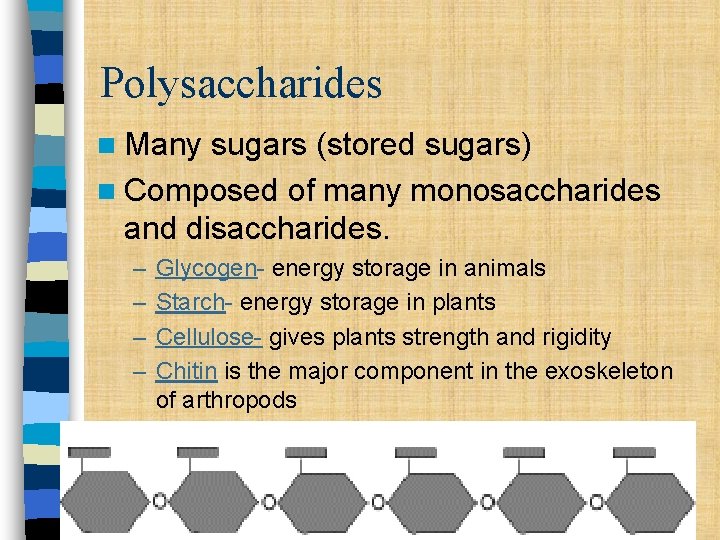
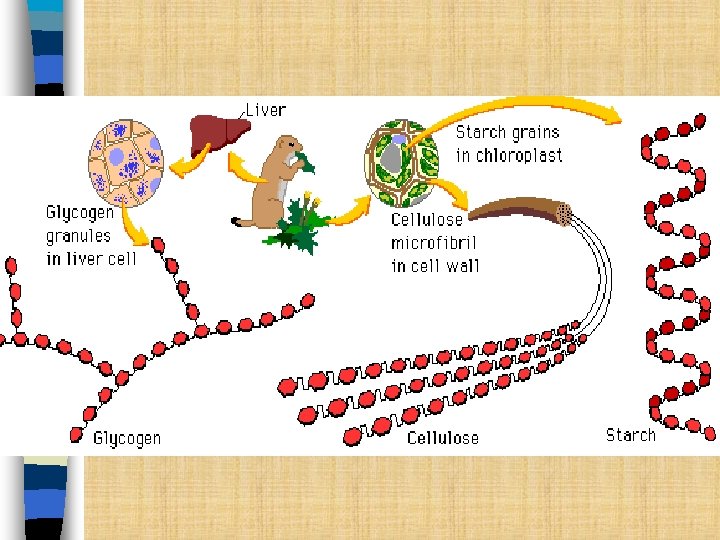
Polysaccharides n Many sugars (stored sugars) n Composed of many monosaccharides and disaccharides. – – Glycogen- energy storage in animals Starch- energy storage in plants Cellulose- gives plants strength and rigidity Chitin is the major component in the exoskeleton of arthropods

Animation- Carbohydrates n http: //nhscience. lonestar. edu/biol/dehyd rat/dehydrat. html
Lipids
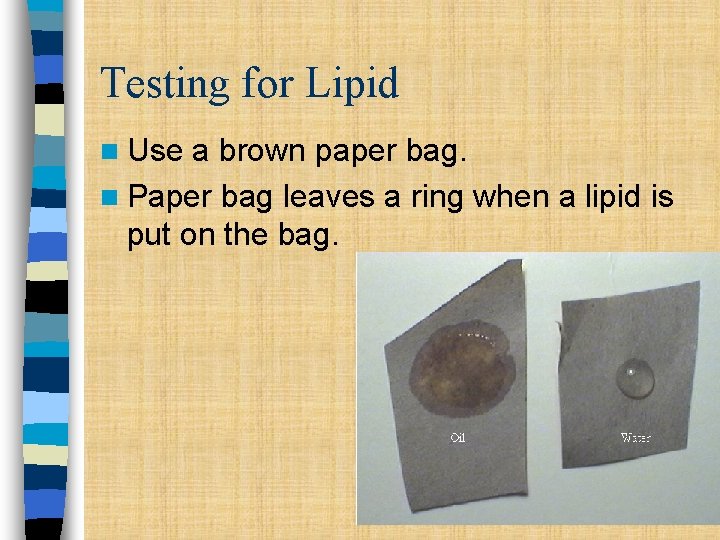
Testing for Lipid n Use a brown paper bag. n Paper bag leaves a ring when a lipid is put on the bag.
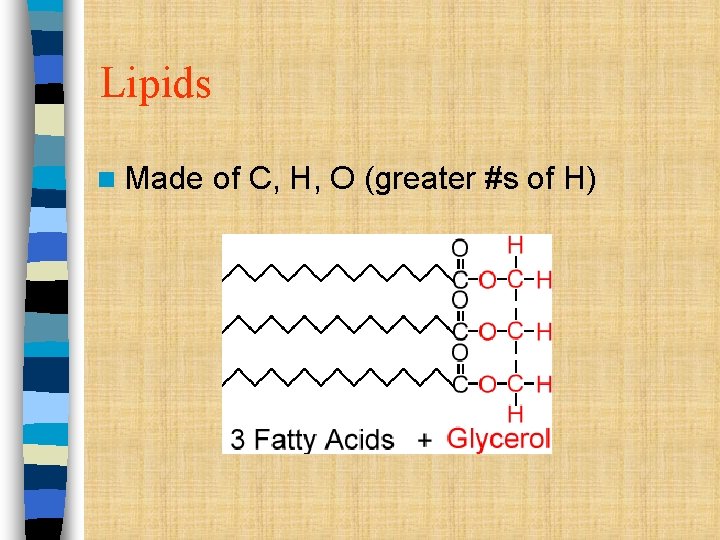
Lipids n Made of C, H, O (greater #s of H)
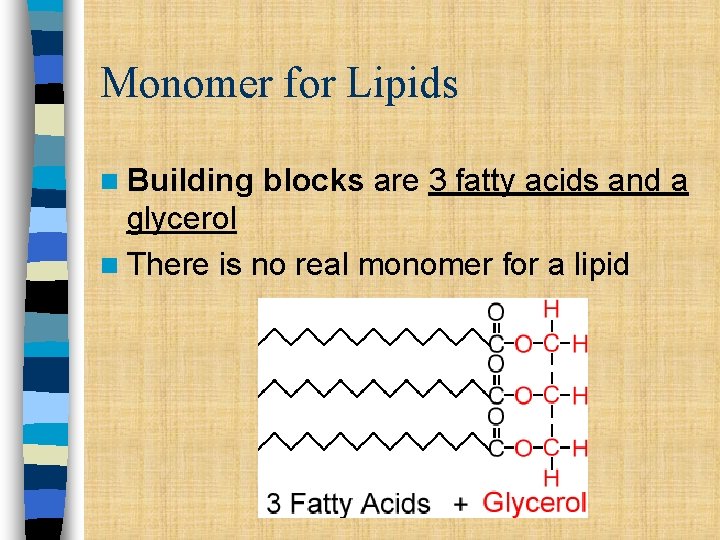
Monomer for Lipids n Building blocks are 3 fatty acids and a glycerol n There is no real monomer for a lipid

Lipids n Uses: insulation, make up parts of cell membranes, protects organs and waterproof coverings n Examples: Fats, oils, and waxes n (won’t dissolve in water)
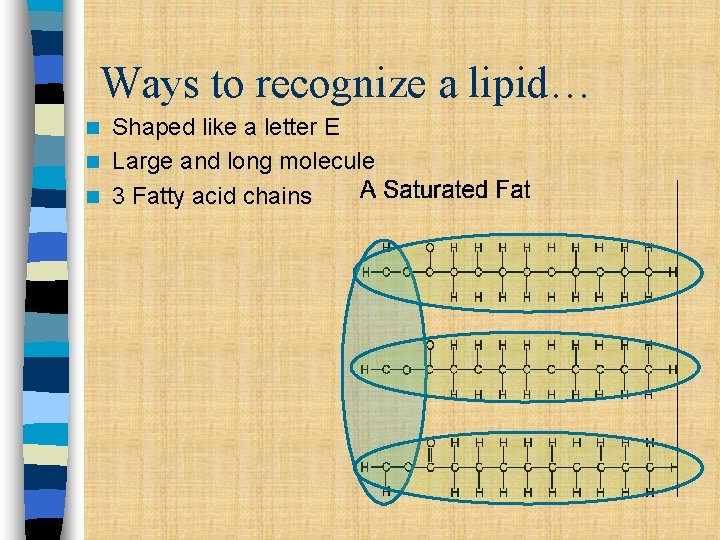
Ways to recognize a lipid… Shaped like a letter E n Large and long molecule n 3 Fatty acid chains n

• Lipids produced by animals are generally solids at room temperature. n Saturated lipids (fats): all carbon in the fatty acid chains are single bonded

Lipids produced by plants are generally liquids at room temperature. n Unsaturated lipid (fats): – the fatty acid component contains C bonded to C using a double bond or a triple bond
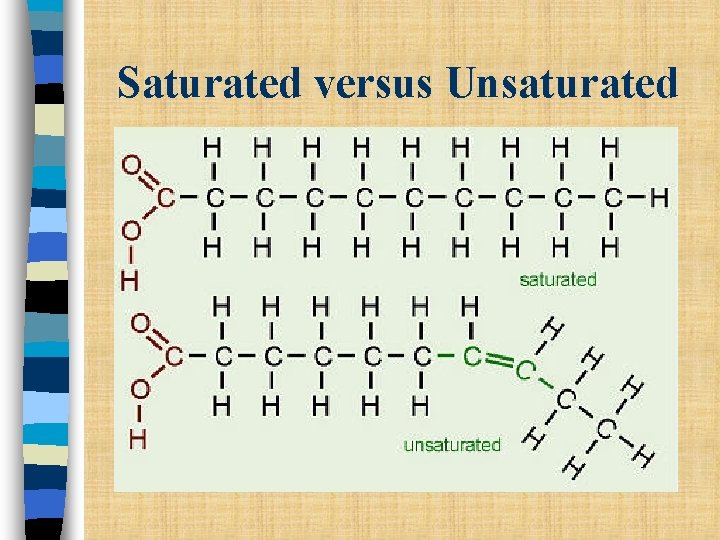
Saturated versus Unsaturated

Lipid formation animation n http: //nutrition. jbpub. com/resources/ani mations. cfm? id=10&debug=0
Proteins

Testing for Protein n Biuret reagent will turn violet in the presence of protein
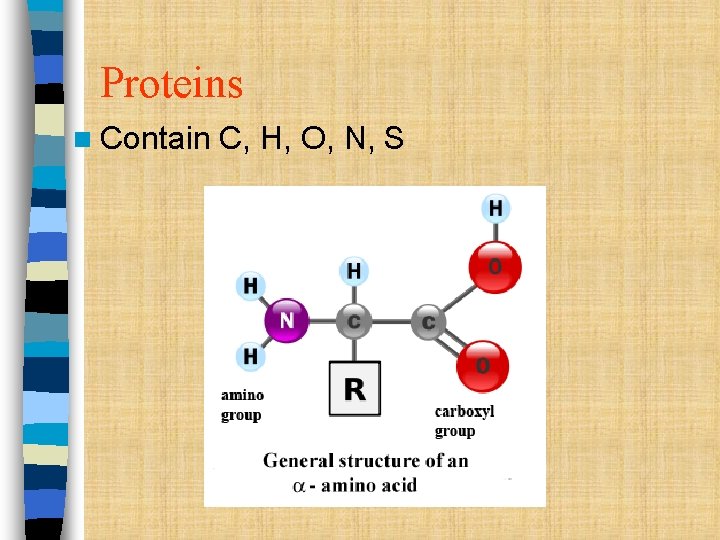
Proteins n Contain C, H, O, N, S
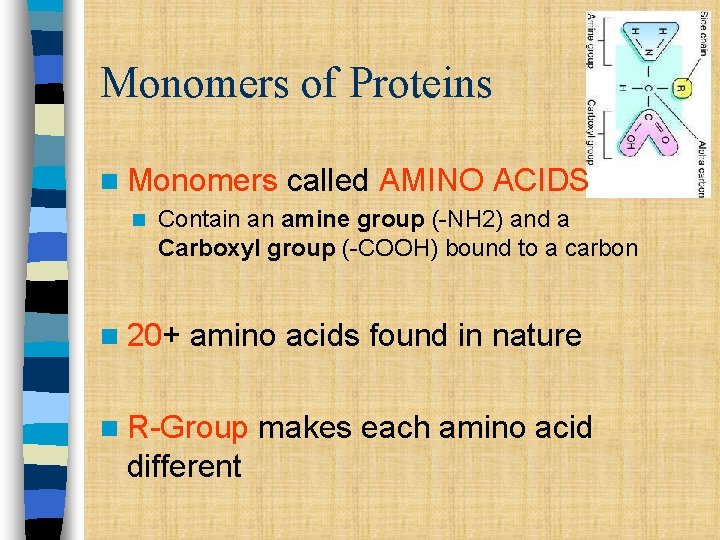
Monomers of Proteins n Monomers n called AMINO ACIDS Contain an amine group (-NH 2) and a Carboxyl group (-COOH) bound to a carbon n 20+ amino acids found in nature n R-Group different makes each amino acid
Protein n Functions-Form bones, muscle, finger nails, hair n Uses: Help transport substances into or out of the cell n Control Reaction Rates- enzymes n Examples: in meat, beans, fish

Animation- Protein n http: //nhscience. lonestar. edu/biol/dehyd rat/dehydrat. html
How can proteins change? n http: //www. sumanasinc. com/webconten t/animations/content/proteinstructure. ht ml
Nucleic Acids
Testing for nucleic acids n Testing n DNA can see if DNA is present… fingerprinting
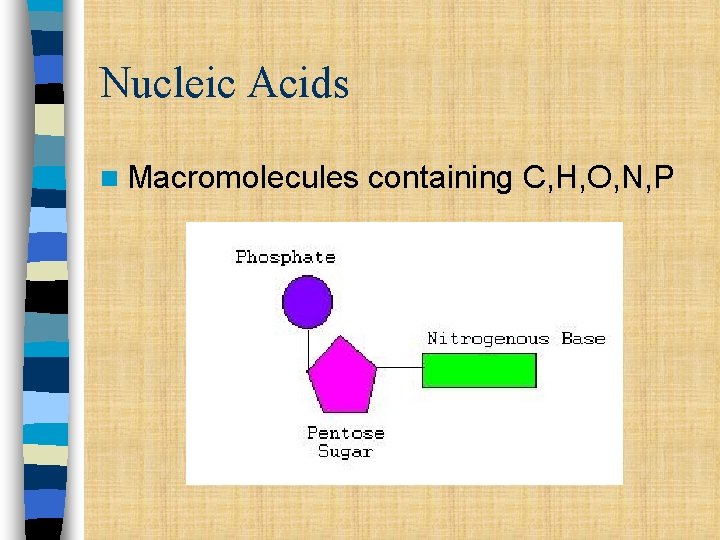
Nucleic Acids n Macromolecules containing C, H, O, N, P
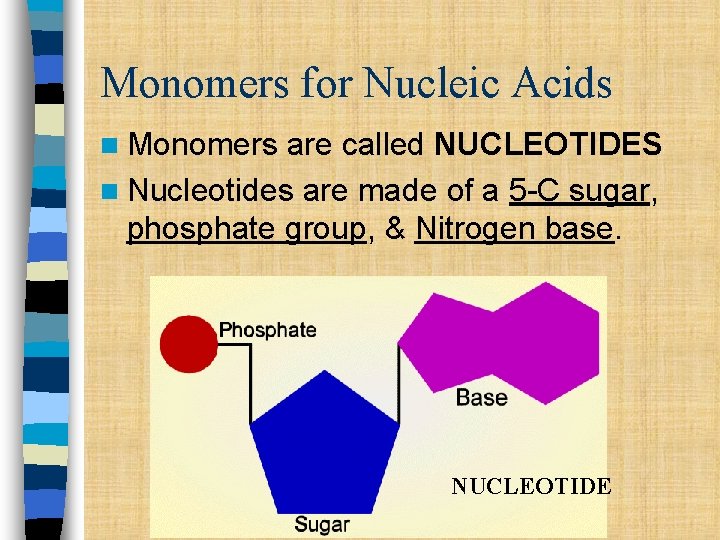
Monomers for Nucleic Acids n Monomers are called NUCLEOTIDES n Nucleotides are made of a 5 -C sugar, phosphate group, & Nitrogen base. NUCLEOTIDE
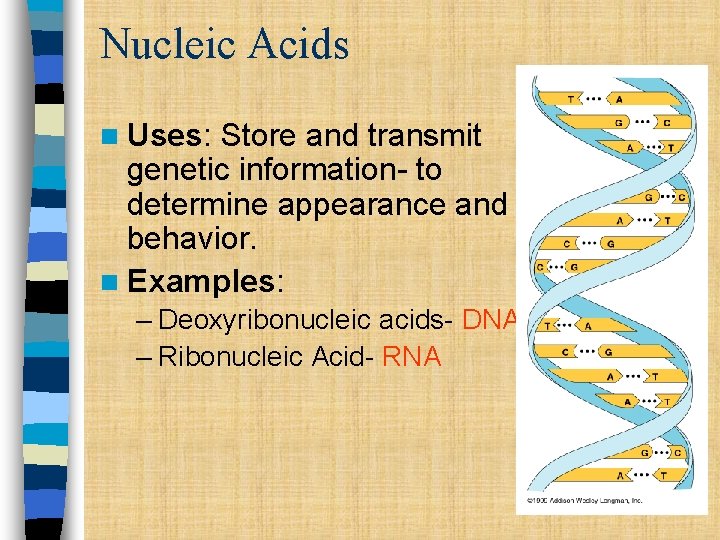
Nucleic Acids n Uses: Store and transmit genetic information- to determine appearance and behavior. n Examples: – Deoxyribonucleic acids- DNA – Ribonucleic Acid- RNA
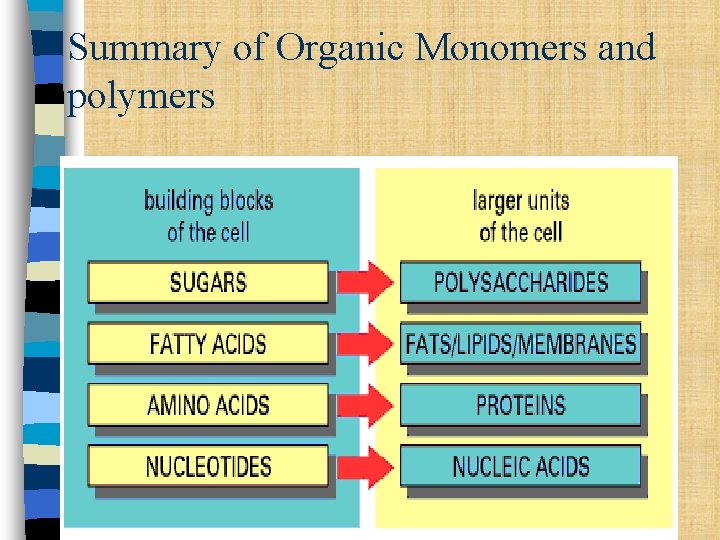
Summary of Organic Monomers and polymers
Enzymes n. A CATALYST -speeds up the rate of a chemical reaction. n ENZYMES -proteins that act as biological catalysts. n Cells use enzymes to speed up chemical reactions that take place in cells.
Enzyme Action n The reactants of enzyme-catalyzed reactions are known as SUBSTRATES. n Enzymes bond to substrates using a lock and key model. n The substrate attaches to the enzyme at the ACTIVE SITE. n If Exposed to extreme temperatures or p. H, an enzyme may DENATURE, or change shape so that the substrate no longer fits and the enzyme will not work.
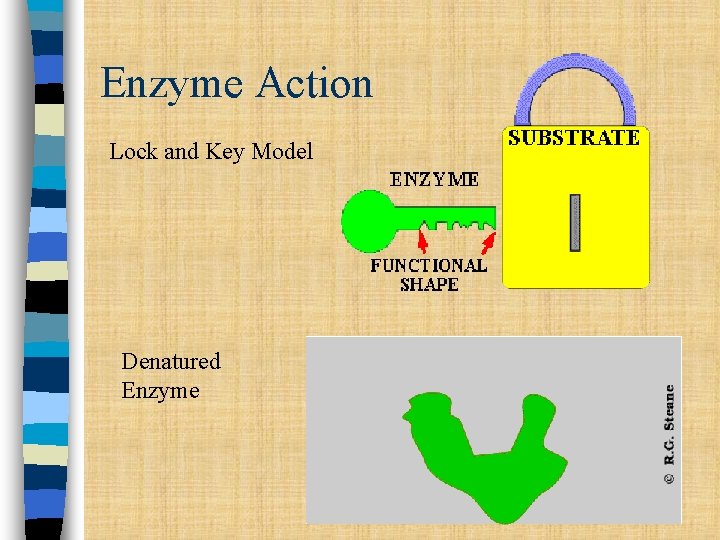
Enzyme Action Lock and Key Model Denatured Enzyme
- Slides: 43