Biochemistry The Chemistry of Life Unit A Workbook
Biochemistry: The Chemistry of Life Unit A Workbook p 1 -6
Biochemicals n n n Cells and the organelles in cells are made out of organic molecules, known as biochemicals. Organic molecules are those that contain carbon. There are four types of biochemicals: 1. 2. 3. 4. Carbohydrates Lipids Proteins Nucleic acids

Chemical bonding n Chemical compounds are held together by different types of bonds: 1. 2. 3. Covalent bonds: when atoms share electrons equally, strong bonding (ex: hold organic molecules together). Ionic bonds: one atom loses an electron another gains an electron and these break apart easily. Polar covalent: molecules with regions that have a slight positive and slight negative natures
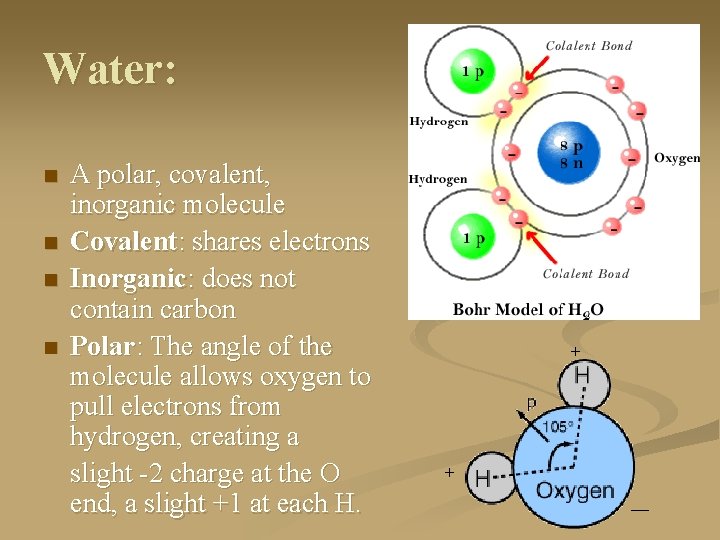
Water: n n A polar, covalent, inorganic molecule Covalent: shares electrons Inorganic: does not contain carbon Polar: The angle of the molecule allows oxygen to pull electrons from hydrogen, creating a slight -2 charge at the O end, a slight +1 at each H. + + __
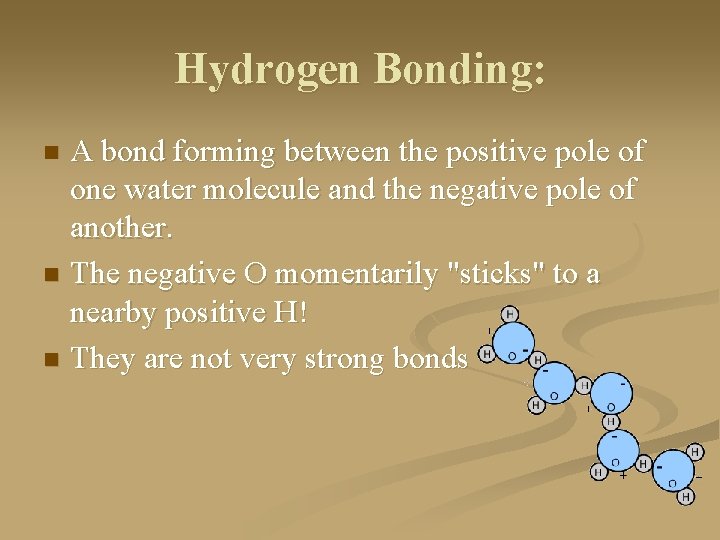
Hydrogen Bonding: A bond forming between the positive pole of one water molecule and the negative pole of another. n The negative O momentarily "sticks" to a nearby positive H! n They are not very strong bonds n

Cohesion vs Adhesion n Cohesion: the tendency of water to stick together n n Provides surface tension, helps pull water up tubes Adhesion: the tendency of water to stick to other surfaces n Helps water flow up small tubes (ie capillaries)

Three properties of water that make it very important to living things: 1. Universal solvent: many compounds dissolve in water. n 2. Importance: Dissolved compounds can be brought to cells (via sap or blood) or move about cell cytoplasm. Temperature Regulator: Large amounts of energy are needed to raise temp of water. n Importance: Water bodies have stable temperatures. Body temps can be maintained. Transfer of heat from warm to cool body parts.

Three properties of water that make it very important to living things: 3. Lubricant: its abundance combined with its properties of adhesion, cohesion, solvation and heat capacity, make water an extremely valuable lubricant n n Example: water in saliva moistens food and lubricates the esophagus during swallowing. Importance: helps move materials from one area of an organism to another with ease.

Acids, Bases and Buffers Acids: molecules that dissociate to release a hydrogen ion (H+1) n Bases: molecules that dissociate to release a Hydroxide ion (OH-1) n n Note: water is neither an acid or a base as it releases both hydrogen and hydroxide ions in equal numbers. So water is said to be neutral.
Acids, Bases and Buffers n p. H: a measure of the free H+1 in a solution. n n n p. H and the human body: n n Can range from a low of 1 (acidic, more H+1 than OH-1) to a high of 14 (basic, more OH-1 than H+1) ph of 7 is neutral (equal amounts of H+1 and OH-1) Muscle tissue: 7. 35 Lungs: 7. 38 Stomach: 2. 5 Any large deviation in these values would hamper the ability of the corresponding organ to function. n This is a major factor in the denaturing or breaking down of enzymes necessary for chemical reactions
Acids, Bases and Buffers prevent significant changes in p. H and maintain homeostasis. n They pick up or release hydrogen (H+1) ions or Hydroxide (OH-1) ions to help maintain the appropriate p. H. n Example: bicarbonate ion HCO 3 -1 act in the digestion and the circulatory system. n
Macromolecules n Cells make their large molecules by joining small organic molecules into chains called polymers. n Polymer = large molecule consisting of many identical or similar molecular units strung together. n Monomer = individual units that serve as building blocks of polymers.
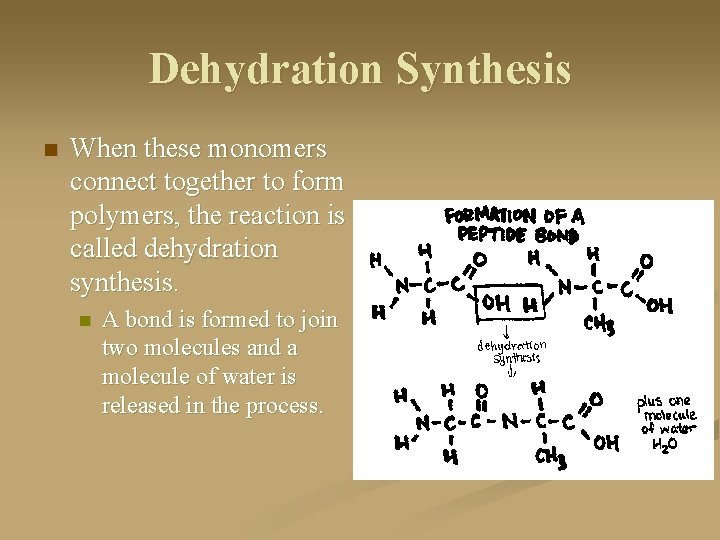
Dehydration Synthesis n When these monomers connect together to form polymers, the reaction is called dehydration synthesis. n A bond is formed to join two molecules and a molecule of water is released in the process.

Hydrolysis The reverse reaction involving the addition of water to break apart polymers into individual unit molecules. n both hydrolysis and dehydration synthesis require enzymes for the reactions to occur. n

Carbohydrates Literally means “hydrates of carbon” n Empirical formula: CH 2 O n Basically for every carbon atom you must have one water molecule n So if you have 5 carbons how many hydrogens and oxygens would you have to have? n 10 hydrgens and 5 oxygens n n Carbohydrates are typically classified according to the number of saccharide (sugar) units they have.
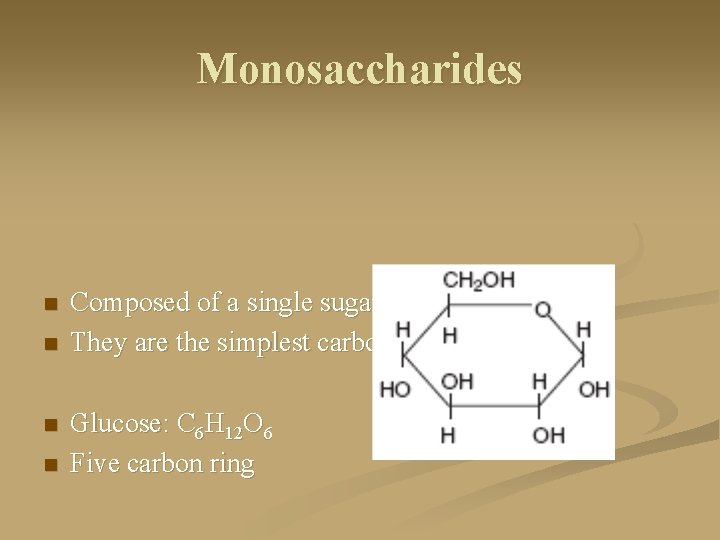
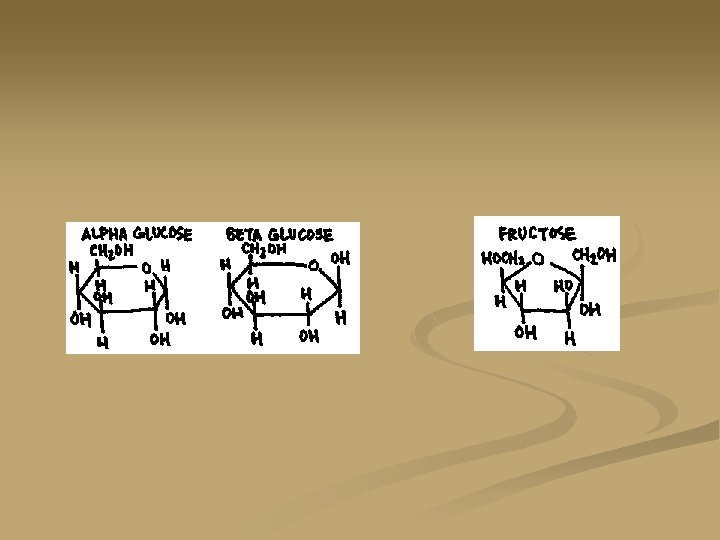
Monosaccharides n n Composed of a single sugar unit They are the simplest carbohydrates Glucose: C 6 H 12 O 6 Five carbon ring
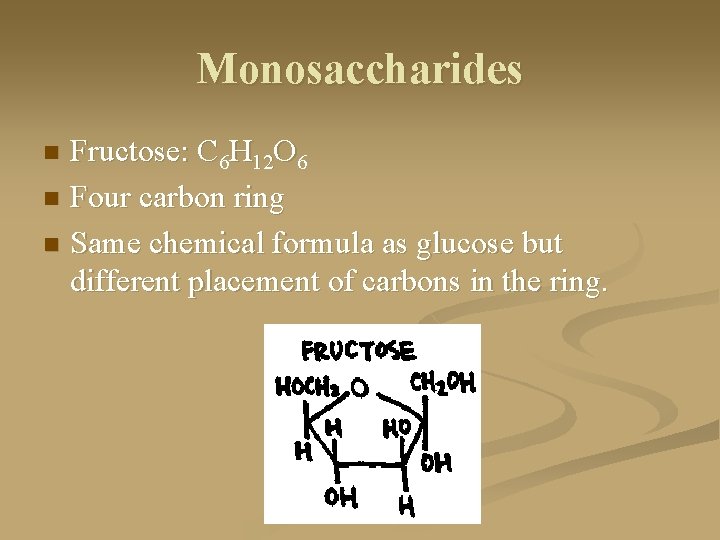
Monosaccharides Fructose: C 6 H 12 O 6 n Four carbon ring n Same chemical formula as glucose but different placement of carbons in the ring. n

Disaccharides n n n When monosaccharides undergo dehydration synthesis to become a double sugar (two sugar units) they are called disaccharides Example: two glucose molecules will join to form a double sugar, or disaccharide, called maltose. Examples: sucrose, table sugar, is a disaccharide formed by joining a fructose and a glucose in dehydration synthsis
Polysaccharides n n n Polysaccharides are composed of three plus sugar units. Since it is formed from repeating units of another molecule it is called a polymer. There are four main kinds of polysaccharides: 1. 2. 3. 4. Starch: storage of glucose in plants Glycogen: storage of glucose in animals Cellulose: cell walls Chitin: exoskeletons of arthropods and cell walls in fungi
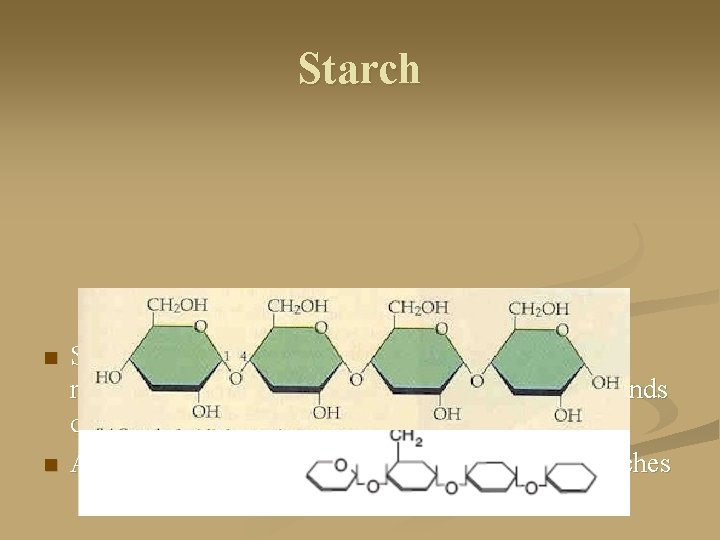
Starch n n Starch is the storage form of sugar in plants. It is made of amylose and amylopectin -- each thousands of glucose units in length A linear polymer that spirals and sometimes branches

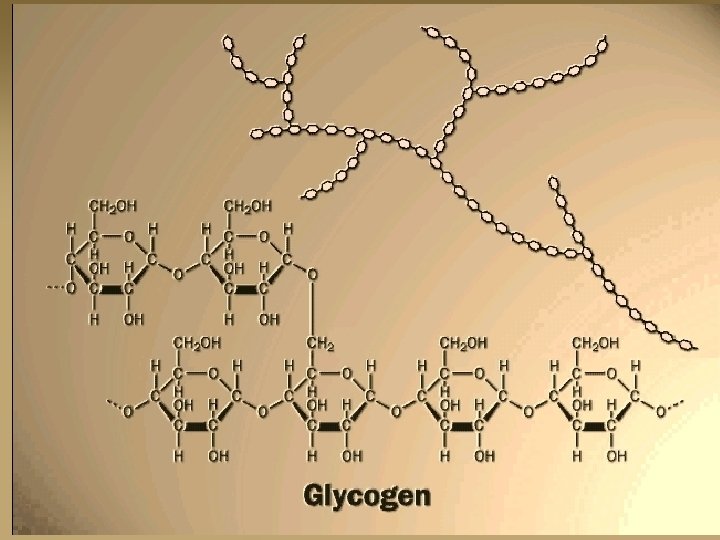
Glycogen n Glycogen is the storage form of sugar in animals. It looks like starch, but with more glucose many branches attached.
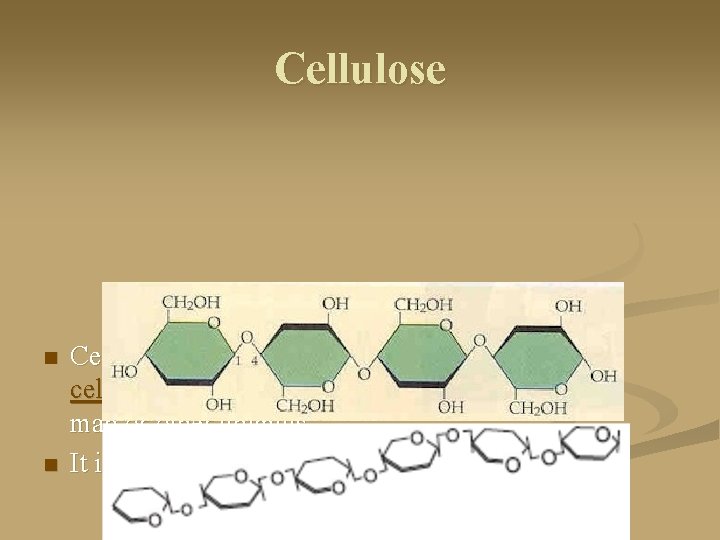
Cellulose n n Cellulose is a structural carbohydrate that forms cell walls in plants. It is not digestible by either man or other animals. It is a linear sequence of glucose molecules.

Lipids: Usually water insoluble (don’t mix freely with polar solvents) n The second most important energy molecules for animals n They include: n Fatty acids n Neutral Fats n Oils n Steroids n Waxes and other special molecules n
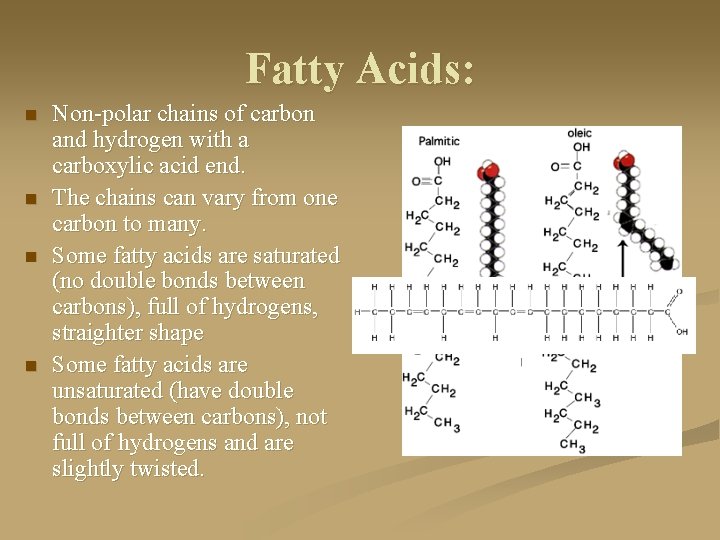
Fatty Acids: n n Non-polar chains of carbon and hydrogen with a carboxylic acid end. The chains can vary from one carbon to many. Some fatty acids are saturated (no double bonds between carbons), full of hydrogens, straighter shape Some fatty acids are unsaturated (have double bonds between carbons), not full of hydrogens and are slightly twisted.

Fatty Acids: Animal fatty acids tend to by more saturated and more solid at room temperatures n While those fatty acids produced by plant tissues are unsaturated, and tend to be more liquid at room temp. (ie: vegetable oil) n

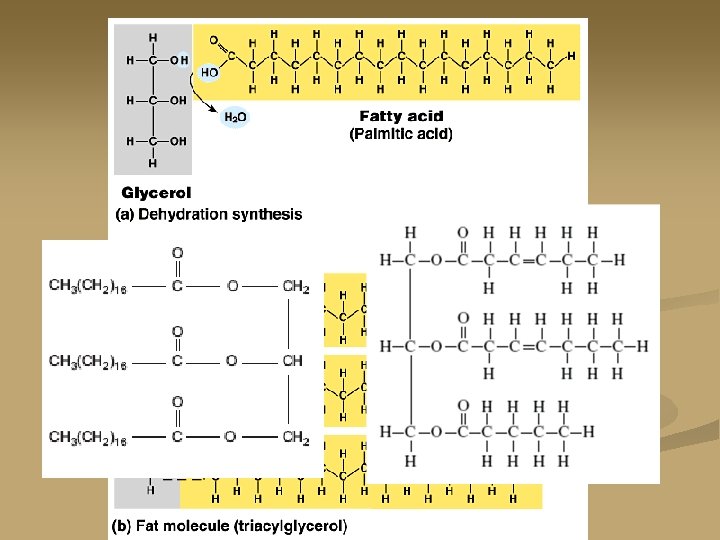
Neutral Fats: n They are produced by dehydration synthesis of one or more fatty acids with a glycerol (an alcohol). monoglyceride: one fatty acid on a glycerol n Diglyceride: two fatty acids on a glycerol n Triglyceride: three fatty acids on a glycerol n
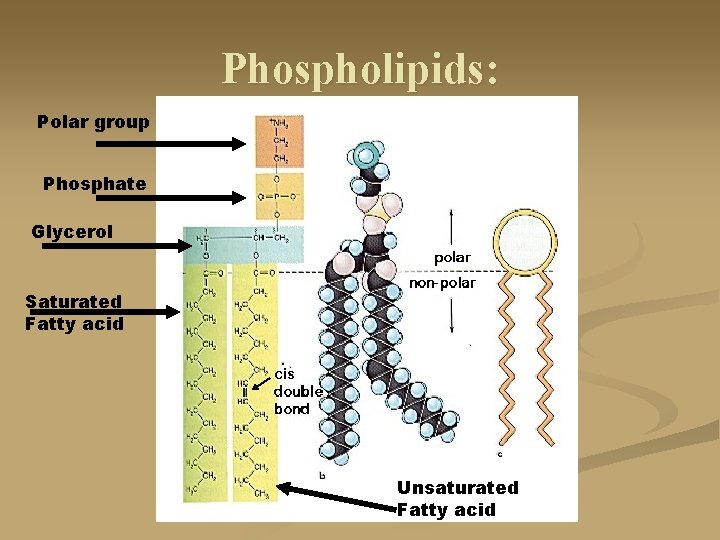
Phospholipids: Polar group Phosphate Glycerol Saturated Fatty acid Unsaturated Fatty acid
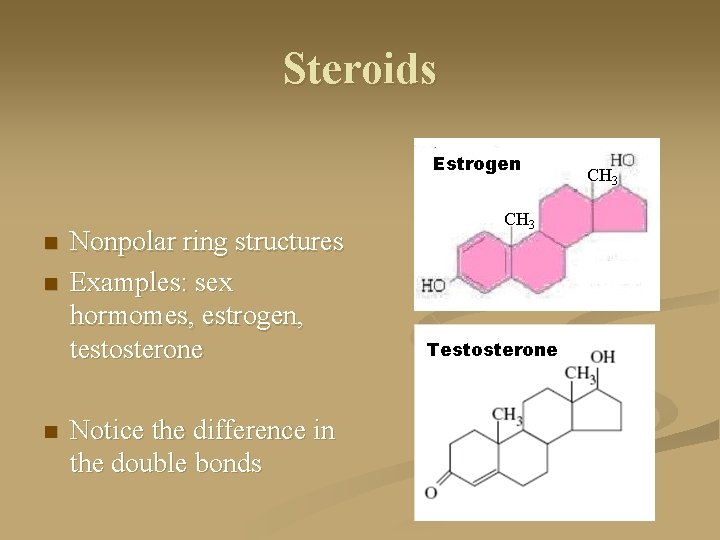
Steroids Estrogen n Nonpolar ring structures Examples: sex hormomes, estrogen, testosterone Notice the difference in the double bonds CH 3 Testosterone CH 3
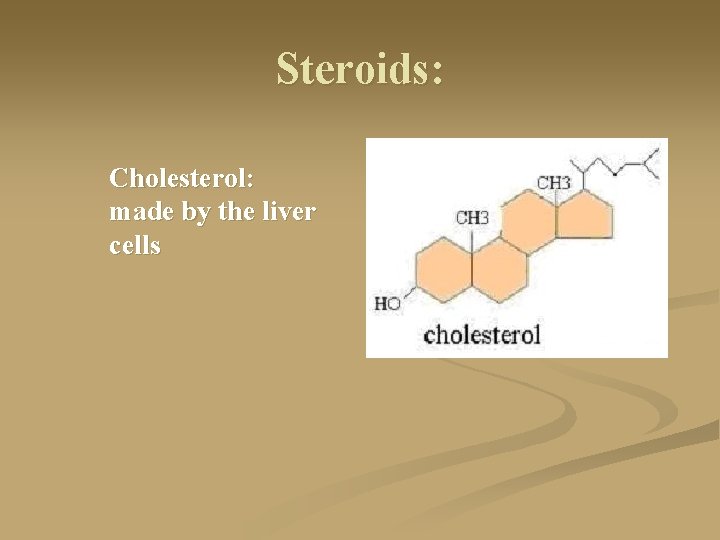
Steroids: Cholesterol: made by the liver cells

Waxes A combination of fatty acids and an alcohol larger than glycerol. n Ex: ear wax n
Proteins: n n Made up of polymers of unit molecules called amino acids Functions: 1. Functional: serve a particular function in body reactions (ie enzymes, antibodies, transportation) n 2. Ex: Hemoglobin or a membrane protein Structural: they form a particular structure fot the body. n Ex: keratin (finger nails or hair) or collagen (connective tissue)
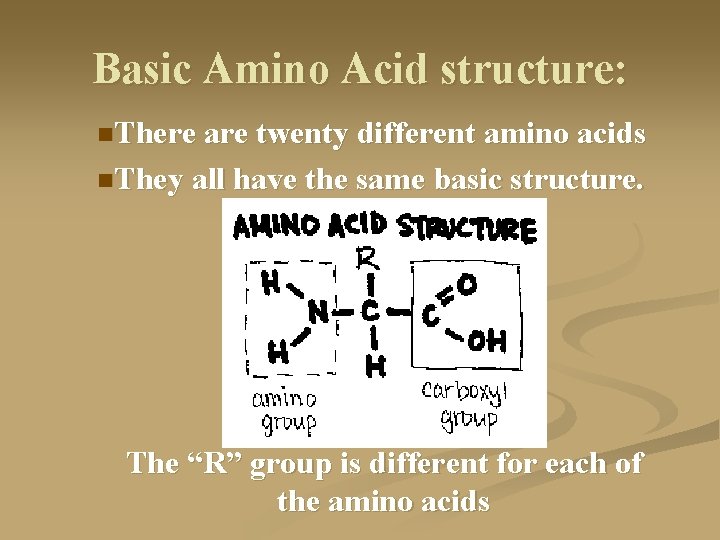
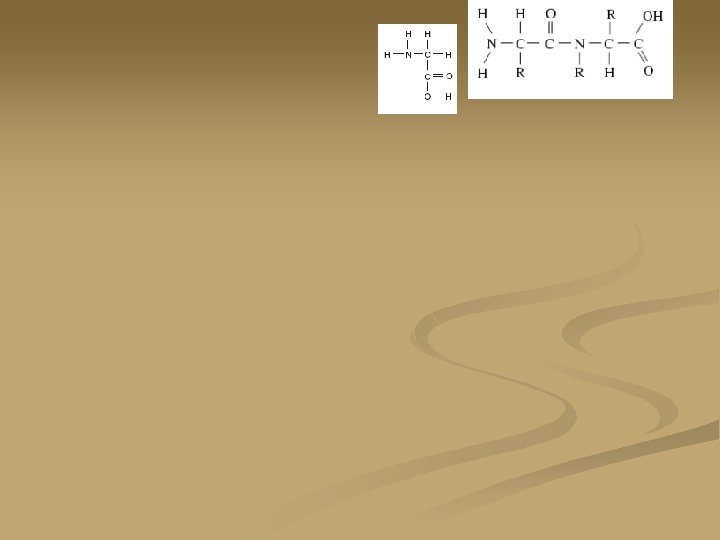
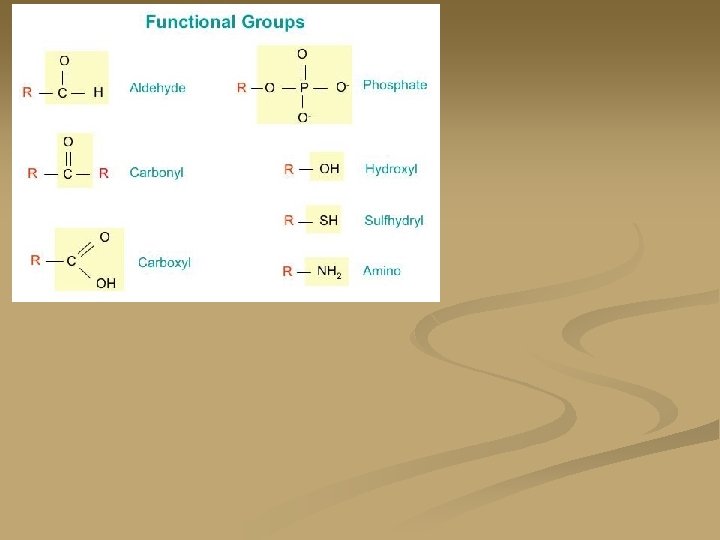
Basic Amino Acid structure: n. There are twenty different amino acids n. They all have the same basic structure. The “R” group is different for each of the amino acids
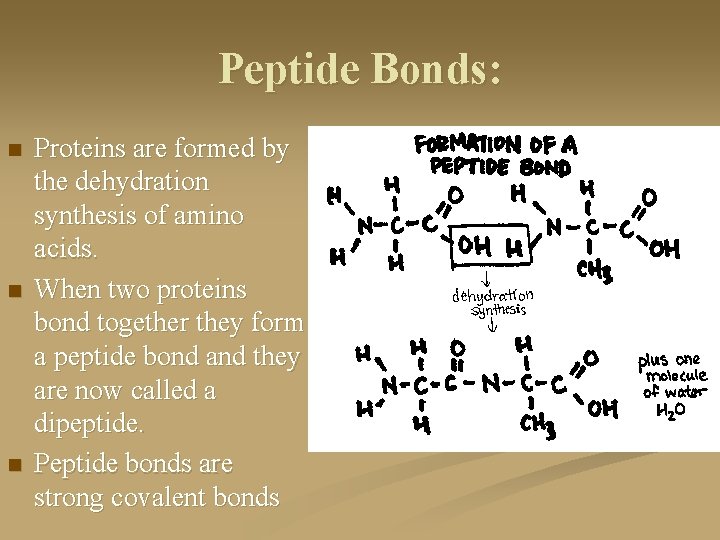
Peptide Bonds: n n n Proteins are formed by the dehydration synthesis of amino acids. When two proteins bond together they form a peptide bond and they are now called a dipeptide. Peptide bonds are strong covalent bonds

Peptide Bonds: When three amino acids are held together by two peptide bonds it is called a tripeptide. n Any more than three amino acids and it is called a polypeptide. n
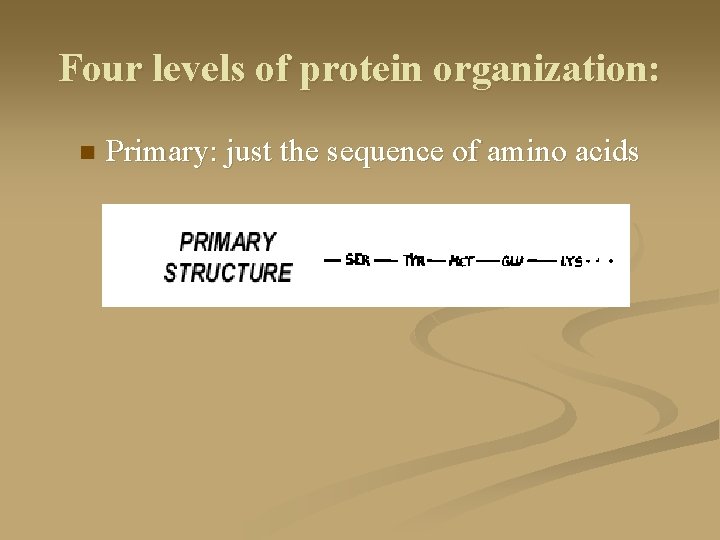
Four levels of protein organization: n Primary: just the sequence of amino acids
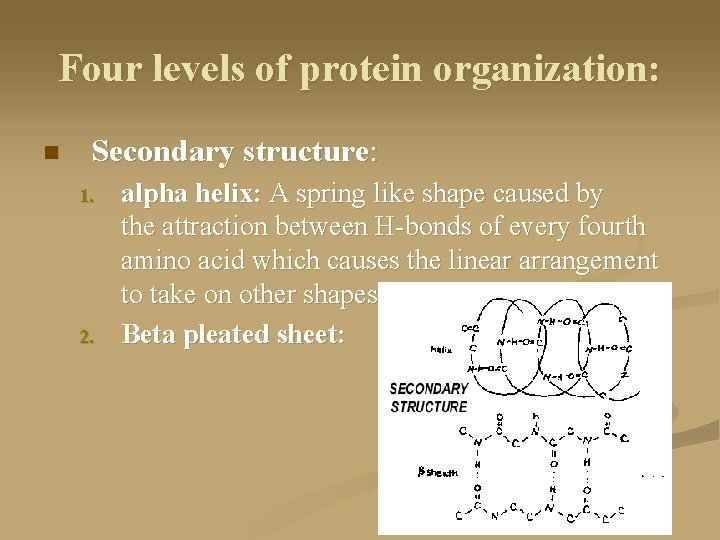
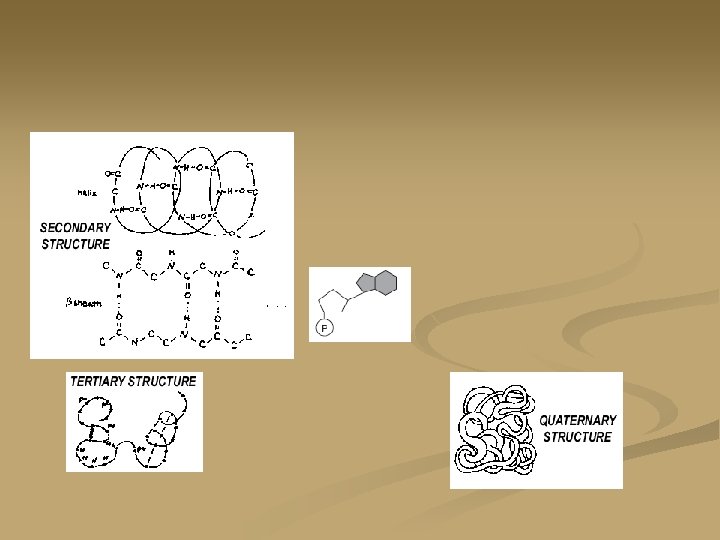
Four levels of protein organization: n Secondary structure: 1. 2. alpha helix: A spring like shape caused by the attraction between H-bonds of every fourth amino acid which causes the linear arrangement to take on other shapes. Beta pleated sheet:
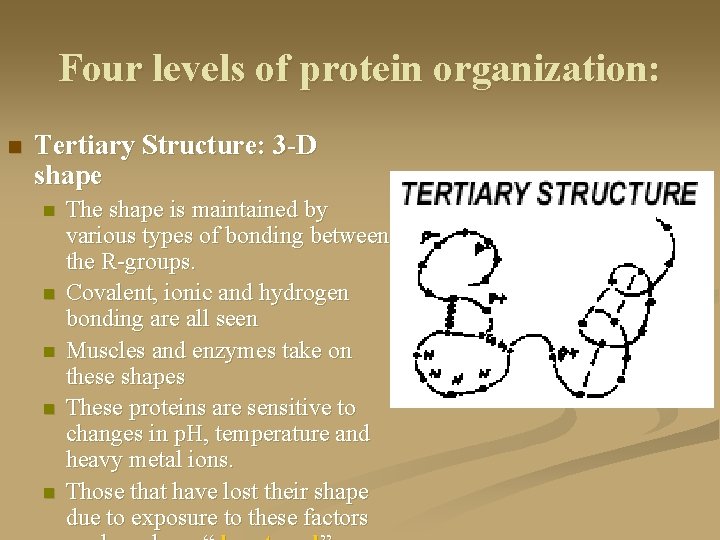
Four levels of protein organization: n Tertiary Structure: 3 -D shape n n n The shape is maintained by various types of bonding between the R-groups. Covalent, ionic and hydrogen bonding are all seen Muscles and enzymes take on these shapes These proteins are sensitive to changes in p. H, temperature and heavy metal ions. Those that have lost their shape due to exposure to these factors
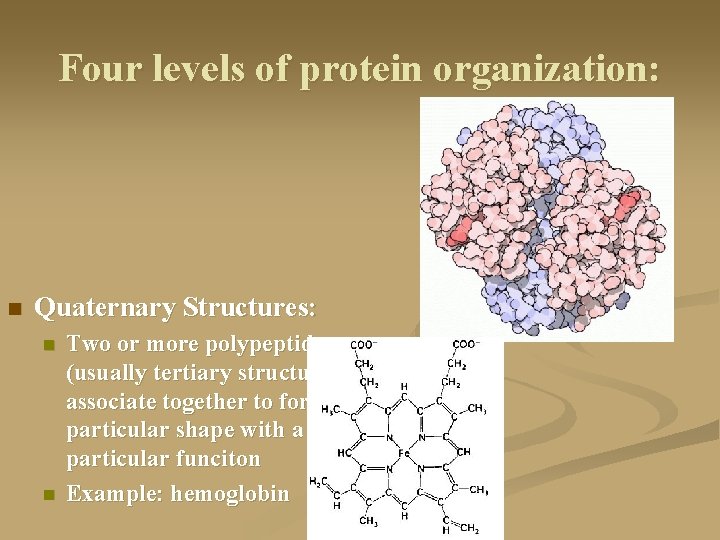
Four levels of protein organization: n Quaternary Structures: n n Two or more polypeptides (usually tertiary structures) associate together to form a particular shape with a particular funciton Example: hemoglobin

Nucleic Acids: n Separated into two types 1. 2. Deoxyribonucleic Acid (DNA) Ribonucleic Acid (RNA) They are both polymers of unit molecules called nucleotides. Each nucleotide is made up of a 1. 2. 3. A sugar A phosphate group Nitrogenous base
DNA vs RNA DNA: n Sugar: Deoxyribose n Nitrogenous Bases: Adenine (A), Thymine (T), Guanine (G), Cytosine (C) n Found only in nucleus RNA: n Sugar: Ribose n Nitrogenous Bases: Adenine (A), Uracil (U), Guanine (G) and Cytosine (C) n Found in the nucleus and the cytoplasm The phosphate group is the same for both DNA and RNA
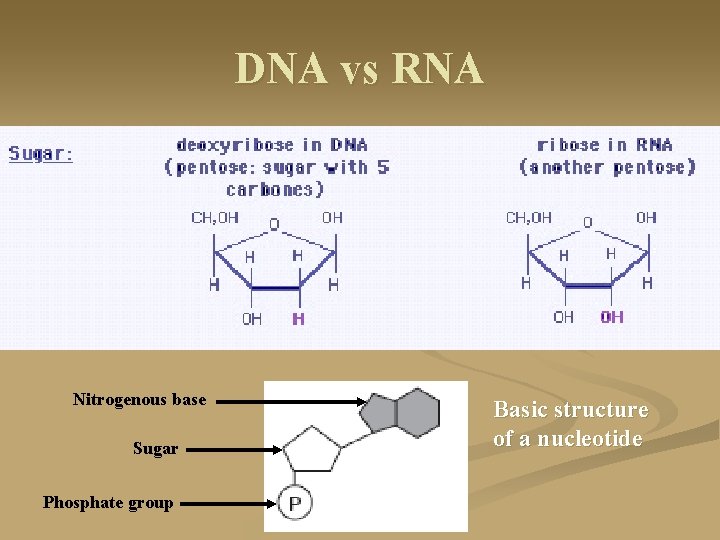
DNA vs RNA Nitrogenous base Sugar Phosphate group Basic structure of a nucleotide
Functions: DNA RNA Function: along with histone proteins, it forms chromosomes, which control cellular activity by containing the instructions for the synthesis of all protiens Function: there are three types of RNA and they are used to make ribosomes, make messages from the nucleus to ribosomes and for the calling of particular amino acids in protein synthesis.
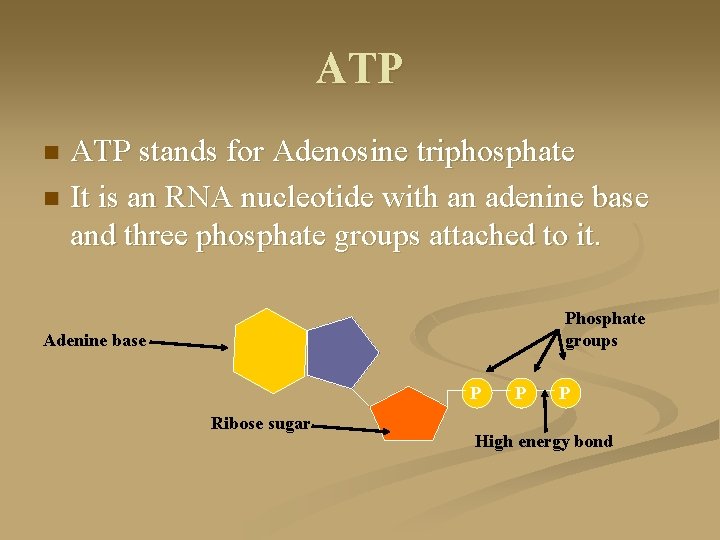
ATP stands for Adenosine triphosphate n It is an RNA nucleotide with an adenine base and three phosphate groups attached to it. n Phosphate groups Adenine base P Ribose sugar P P High energy bond

Review questions Workbook page 6 -9 n Quiz next class (Wednesday) on proteins and nucleic acids n Test next Wednesday all biochemistry n
- Slides: 50