Biochemistry The Chemical Composition of Living Matter Suzanne

Biochemistry The Chemical Composition of Living Matter Suzanne D'Anna 1
Two principal Classes of Compounds Found in the Body organic compounds l inorganic compounds l Suzanne D'Anna 2
Organic Compounds contain carbon and hydrogen - carbon is a unique element because it has 4 electrons in valence shell enabling it to combine with many different atoms - large, covalently bonded molecules l Examples: - carbohydrates, proteins, nucleic acids l Suzanne D'Anna 3
Inorganic Compounds lack hydocarbons l smaller simpler molecules l Examples: - water, oxygen, carbon dioxide salts, some acids and bases l Suzanne D'Anna 4
Water most important - 2/3 body weight Why is water so vital? l high heat capacity l excellent solvent l important reactant l lubricant l protective l transportation l Suzanne D'Anna 5
Salt most plentiful body salts contain calcium and phosphorus l found mostly in bone and teeth l ions of salts provide essential chemical elements l Example: - ionic calcium (Ca++) - essential for nerve impulses l Suzanne D'Anna 6

Acids electrolytes l compounds which ionize and dissociate in H 20 liberating hydrogen ions (H+) l conduct electrical current l sour taste l dissolve and burn l Suzanne D'Anna 7

Bases electrolytes l any molecule which can combine with hydrogen ions l proton (H+) acceptors l help maintain stable p. H of body fluids l bitter taste, feel slippery l Example: - sodium bicarbonate l Suzanne D'Anna 8
p. H Scale based on the number of hydrogen ions is solution l measure of acid or base level l 0 - 6. 9 acid l 7. 1 -14 base l at p. H 7 hydrogen and hydroxyl ions are equal (neutral) l Suzanne D'Anna 9
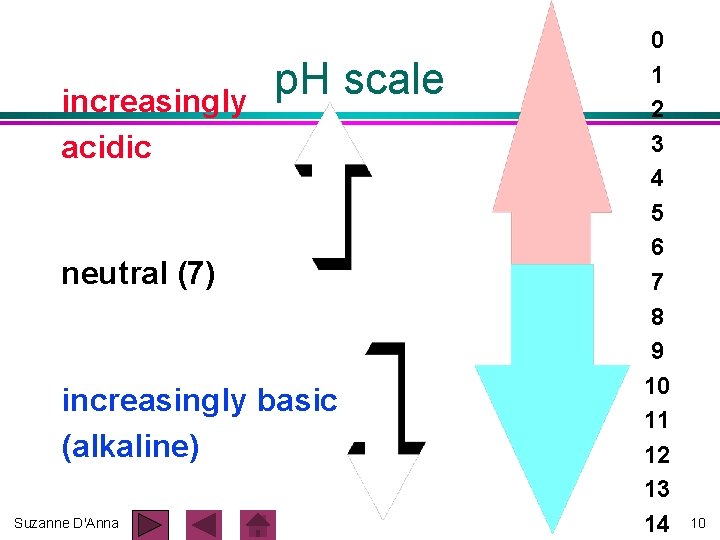
increasingly acidic p. H scale neutral (7) increasingly basic (alkaline) Suzanne D'Anna 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 10
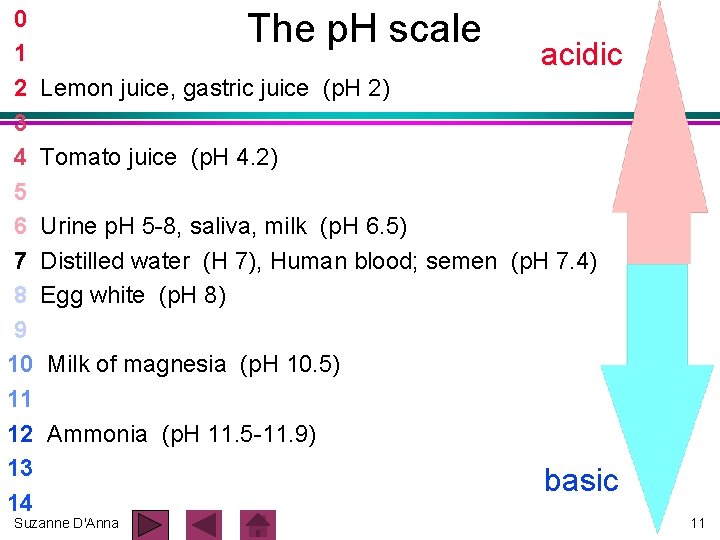
0 1 acidic 2 Lemon juice, gastric juice (p. H 2) 3 4 Tomato juice (p. H 4. 2) 5 6 Urine p. H 5 -8, saliva, milk (p. H 6. 5) 7 Distilled water (H 7), Human blood; semen (p. H 7. 4) 8 Egg white (p. H 8) 9 10 Milk of magnesia (p. H 10. 5) 11 12 Ammonia (p. H 11. 5 -11. 9) 13 basic 14 The p. H scale Suzanne D'Anna 11
Organic Compounds contain carbon and hydrogen l carbon molecules react with many other carbon atoms to form large chains and rings with different molecules l 2 -3% of total body weight l Suzanne D'Anna 12

Carbohydrates are sugars or starches l provide most of the energy used by cells l some carbohydrates are converted into other substances which are used to build structures and to generate ATP. l if not used immediately for ATP synthesis carbohydrates are converted into fat or glycogen l Suzanne D'Anna 13

Carbohydrates (cont. ) monosaccharides - simple sugar - glucose, fructose, galactose l disaccharides - double sugar - maltose, sucrose, and lactose l polysaccharides - many sugars - glycogen, hyaluronic acid, (provides for energy for sperm) and condroitin sulfate l Suzanne D'Anna 14

ATP (adenosine triphosphate) provides chemical energy l necessary for life processes l energy is released as glucose and stored in bonds of ATP l when bonds are broken down (hydrolysis) energy can then be used l Suzanne D'Anna 15
Lipids organic compounds l 18%-25% of body weight l fewer covalent bonds l most are insoluble in H 2 O l fat-marbled meats, egg yolks, milk, oils l Suzanne D'Anna 16

Lipids l (cont. ) neutral fats (triglycerides) - most common in the body - all excess food is converted into triglycerides and stored in fat cells (adipose tissue) as an energy source - adipose tissue insulates and protects organs Suzanne D'Anna 17
Lipids l (cont. ) phospholipids (compound lipids) - contain phosphorus - interact with H 2 O - major component of cell membranes - help in transport of lipids in plasma - found in high concentrations in nerves and brain tissue Suzanne D'Anna 18

Lipids l (cont. ) steroids - cholesterol is most important molecule in steroids - sex hormones, cortisol, bile salts, and vitamin D Suzanne D'Anna 19
Proteins complex in structure l composed of amino acids l larger range of functions than carbohydrates and lipids l normal lean adult is 12 -18% proteins l Suzanne D'Anna 20
Functions of Proteins structural - collagen fibers are found in connective tissues, keratin is in hair and skin l regulatory - many hormones that regulate are proteins l contractile - actin and myosin are protein filaments found in all muscle cells l Suzanne D'Anna 21
Functions of Proteins (cont. ) immunological - antibodies are proteins l transport - hemoglobin carries oxygen and carbon dioxide, lipoproteins carry lipids l catalytic - enzymes alter rate of reactions l Suzanne D'Anna 22
Functional Proteins l antibodies - bind with and inactivate bacteria, toxins, and viruses - function in immune response - help protect body from foreign substances Suzanne D'Anna 23
Functional Proteins l (cont. ) hormones - help regulate growth and development - insulin - regulates blood sugar levels - guide neuron growth Suzanne D'Anna 24
Functional Proteins l (cont. ) transport proteins - hemoglobin transport of oxygen in blood - iron transport Suzanne D'Anna 25
Functional Proteins l (cont. ) contractile proteins - aid in muscle contraction and body movement - aid in cell division, movement and sperm propulsion toward the egg Suzanne D'Anna 26
Functional Proteins l (cont. ) catalyst (enzymes) - essential to almost every biochemical reaction in the body - increase rate of chemical reactions Suzanne D'Anna 27

Nucleic Acids Suzanne D'Anna 28
Nucleic Acids make up genes which provide blueprint for life l determine organism type l direct growth and development l dictate protein structure l composed of C, O, H, N, and Ph l largest biological molecules in the body l Suzanne D'Anna 29
Nucleotides nucleotides are building blocks (monomers) of nucleic acids formed by C, O, H, N, and P, which are bound together l Three main parts: - nitrogenous base (organic base) - a phosphate group - a five-carbon sugar (ribose or deoxyribose) l Suzanne D'Anna 30
Two Major Types of Nucleic Acids DNA (deoxyribonucleic acid) l RNA (ribonucleic acid) l Suzanne D'Anna 31
DNA (deoxyribonucleic acid) genetic material found in cell nucleus l replicates self before cell divides ensuring genetic material in every cell is identical l provides instruction for every protein building block in the body l Suzanne D'Anna 32
DNA (cont. ) long double chain of nucleotides l held together by hydrogen bonds l ladder-like molecule l entire molecule is coiled into a spiral structure called a double helix l Suzanne D'Anna 33
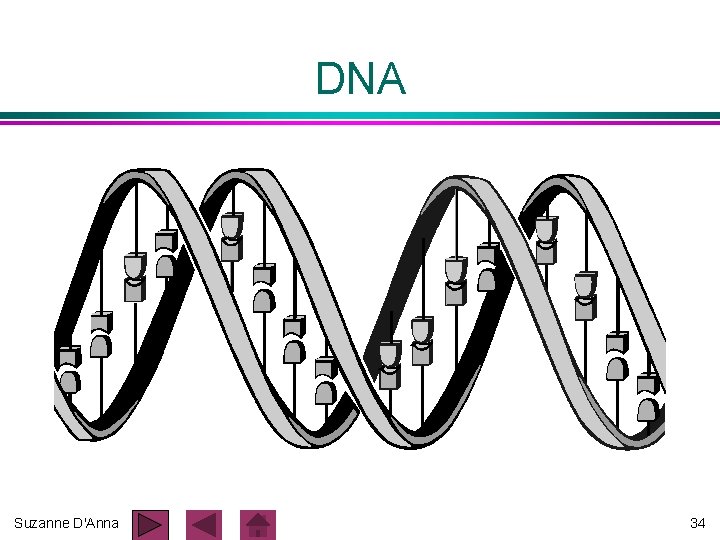
DNA Suzanne D'Anna 34
RNA (ribonucleic acid) located outside nucleus l single nucleotide strand l carries out orders of DNA l three types of RNA - messenger RNA (m. RNA) - transfer RNA (t. RNA) - ribosomal RNA (r. RNA) l Suzanne D'Anna 35
Messenger RNA (m. RNA) 5% of RNA l carries information from nucleus to the cytosol l it is used as template for protein synthesis l Suzanne D'Anna 36
Transfer RNA (t. RNA) 15% of RNA l carries specific amino acids to r. RNA attached to ribosomes l Suzanne D'Anna 37
Ribosomal RNA (r. RNA) 80% of RNA l a component of the ribosomes - ribosomes are structures that are the site of protein synthesis l Suzanne D'Anna 38
- Slides: 38