Biochemistry Standard B3 The student will demonstrate an
Biochemistry Standard: B-3 The student will demonstrate an understanding of the flow of energy within and between living systems. Indicators: B-2. 8, B-3. 4, 5 Chapter 2 – Chemistry of Life
B-3. 4, 5: Organic Molecules (sec 2. 3) 1. What molecule makes up the majority of a cell? 2. What is the difference between organic and inorganic molecules? (give examples of each) 3. Explain the relationship between monomers and polymers. 4. What is the caloric value of proteins, carbohydrates and fats (lipids) ? 5. What are the functions of proteins, carbohydrates, and fats (lipids) in the human body? (include examples)
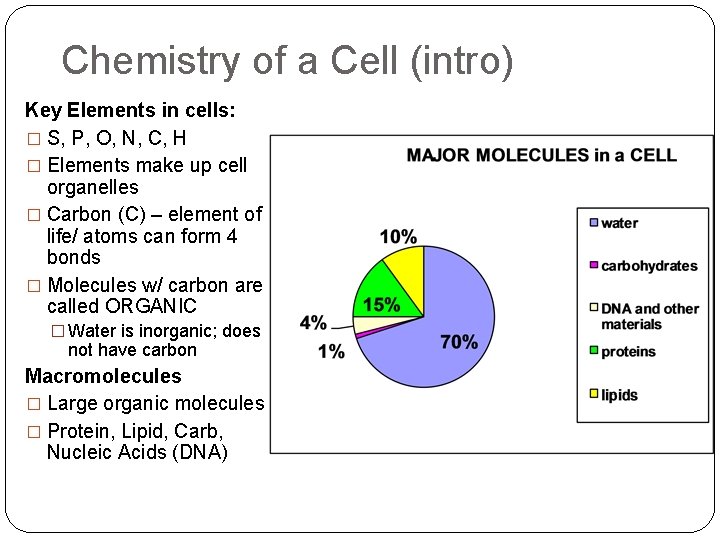
Chemistry of a Cell (intro) Key Elements in cells: � S, P, O, N, C, H � Elements make up cell organelles � Carbon (C) – element of life/ atoms can form 4 bonds � Molecules w/ carbon are called ORGANIC � Water is inorganic; does not have carbon Macromolecules � Large organic molecules � Protein, Lipid, Carb, Nucleic Acids (DNA)
Organic vs. Inorganic �Inorganic – do not contain Carbon �Ex: water, salt, minerals, vitamins �Organic – contain Carbon �Organic chemistry is the study of compounds that contain carbon – carbon has 4 valence electrons which means it can form 4 covalent bonds – carbon bonds with many elements (hydrogen, oxygen, phosphorus, sulfur, nitrogen) �Carbon can form single, double, or triple bonds with other carbon atoms creating millions of different complex structures – these complex molecules make up living things �Ex: Macromolecules (large organic molecules) �Carbohydrates �Lipids �Proteins �Nucleic Acids
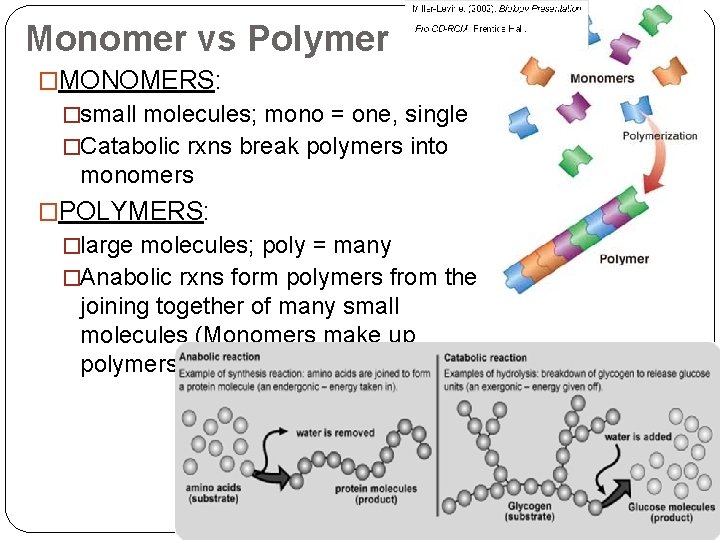
Monomer vs Polymer �MONOMERS: �small molecules; mono = one, single �Catabolic rxns break polymers into monomers �POLYMERS: �large molecules; poly = many �Anabolic rxns form polymers from the joining together of many small molecules (Monomers make up polymers)
Macromolecules FLIPBOOK Resource Download Biology HD Cassiopeia Project collection to i. Tunes. U (ipad) and watch the following videos “Carbohydrates” “Lipids” “Proteins”
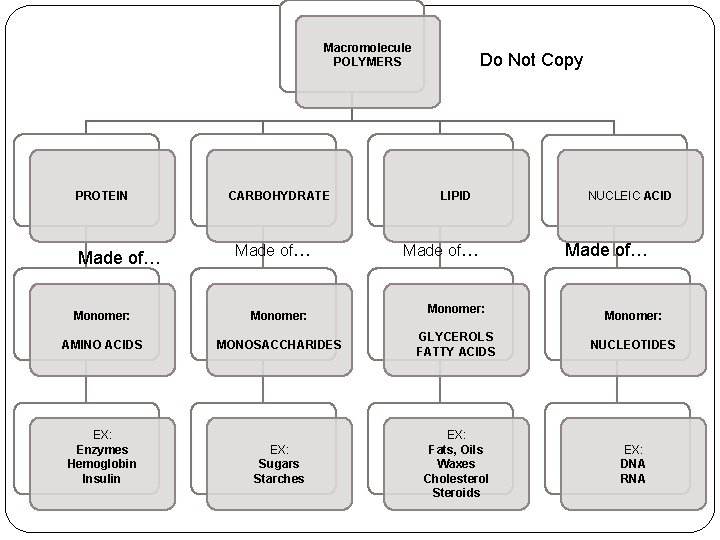
Macromolecule POLYMERS PROTEIN Made of… CARBOHYDRATE Made of… Do Not Copy LIPID Made of… Monomer: NUCLEIC ACID Made of… Monomer: AMINO ACIDS MONOSACCHARIDES GLYCEROLS FATTY ACIDS NUCLEOTIDES EX: Sugars Starches EX: Fats, Oils Waxes Cholesterol Steroids EX: DNA RNA EX: Enzymes Hemoglobin Insulin Monomer:
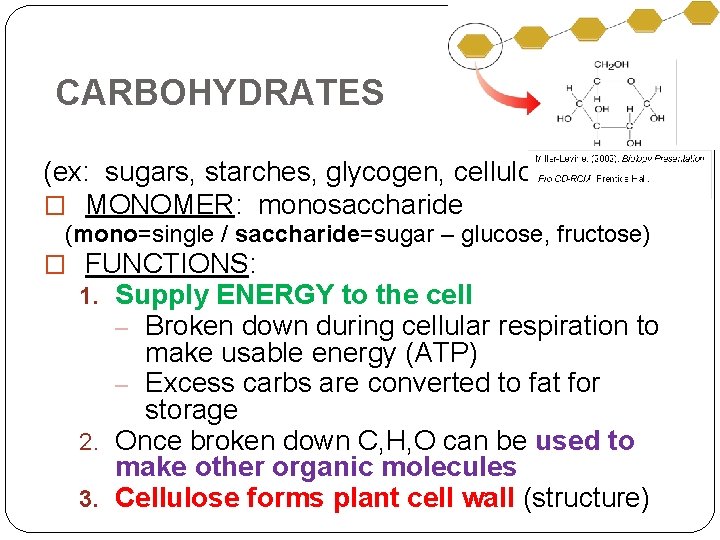
CARBOHYDRATES (ex: sugars, starches, glycogen, cellulose) � MONOMER: monosaccharide (mono=single / saccharide=sugar – glucose, fructose) � FUNCTIONS: 1. Supply ENERGY to the cell – Broken down during cellular respiration to make usable energy (ATP) – Excess carbs are converted to fat for storage 2. Once broken down C, H, O can be used to make other organic molecules 3. Cellulose forms plant cell wall (structure)
LIPIDS (ex: Fats, Oils, Cholesterol, Steroids, & Waxes) � MONOMER: fatty acids & glycerol � FUNCTIONS: 1. STORE ENERGY: provide energy reserve – Can be used for ENERGY if carbohydrates 2. 3. 4. 5. are not available INSULATION: conserve heat Phospholipids – make up cell membranes Cholesterol – part of cell membrane makes steroids (Ex: testosterone, estrogen)
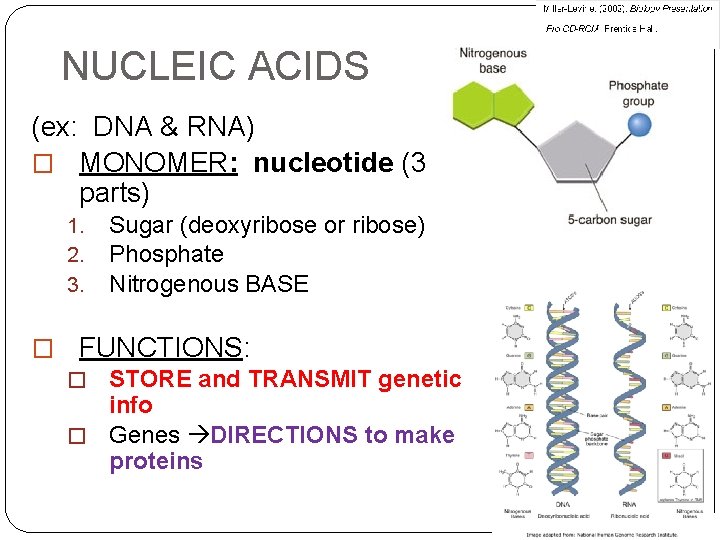
NUCLEIC ACIDS (ex: DNA & RNA) � MONOMER: nucleotide (3 parts) 1. 2. 3. Sugar (deoxyribose or ribose) Phosphate Nitrogenous BASE � FUNCTIONS: � STORE and TRANSMIT genetic info � Genes DIRECTIONS to make proteins
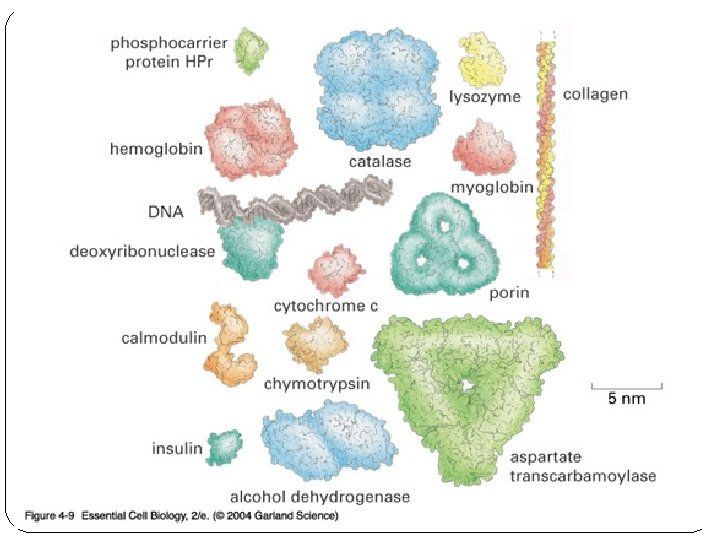
PROTEINS (Ex: digestive enzymes, insulin, collagen, antibodies, muscle) � MONOMER: amino acid �only 20 types of amino acids �Amino acids joined by peptide bonds to create 3 -D proteins �Shape of protein relates to function � Red blood cells contain the protein Hemoglobin – shape of Hemoglobin allows it to carry oxygen molecules � FUNCTIONS (lots and lots of functions) �Building Block of Cell (most abundant molecule) �CONTROL RXN RATES – enzymes (digestive enzymes) �REGULATATORY proteins (insulin) �STRUCTURAL proteins (keratin, collagen, connective tissue) �FIGHT disease (antibodies) �TRANSPORT proteins (hemoglobin) �Contractile proteins (muscle contractions)
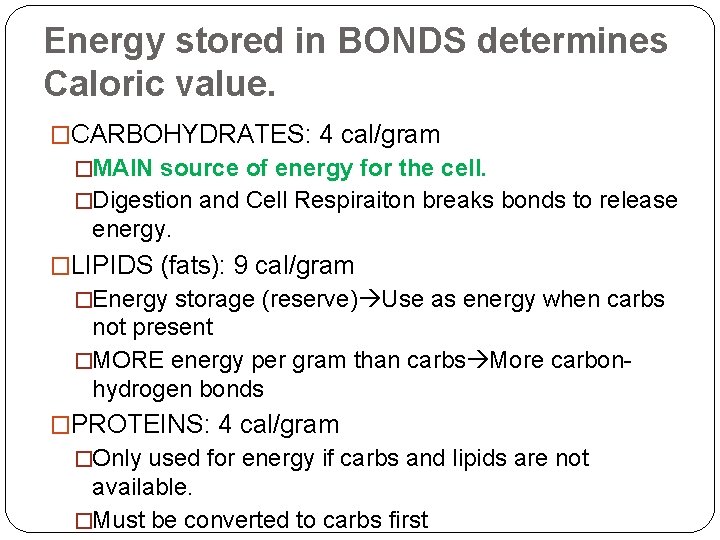
Energy stored in BONDS determines Caloric value. �CARBOHYDRATES: 4 cal/gram �MAIN source of energy for the cell. �Digestion and Cell Respiraiton breaks bonds to release energy. �LIPIDS (fats): 9 cal/gram �Energy storage (reserve) Use as energy when carbs not present �MORE energy per gram than carbs More carbonhydrogen bonds �PROTEINS: 4 cal/gram �Only used for energy if carbs and lipids are not available. �Must be converted to carbs first
B-2. 8: Biochemical Reactions (sec 2. 2, 4) 1. Explain the relationship between chemical 2. 3. 4. 5. reactions and energy. Explain the importance of water in maintaining homeostasis. (high specific heat & buffer) What is a catalyst & How do enzymes affect the rate of reactions? LIST 5 properties of enzymes: How is an enzyme reaction like a “lock and key”?
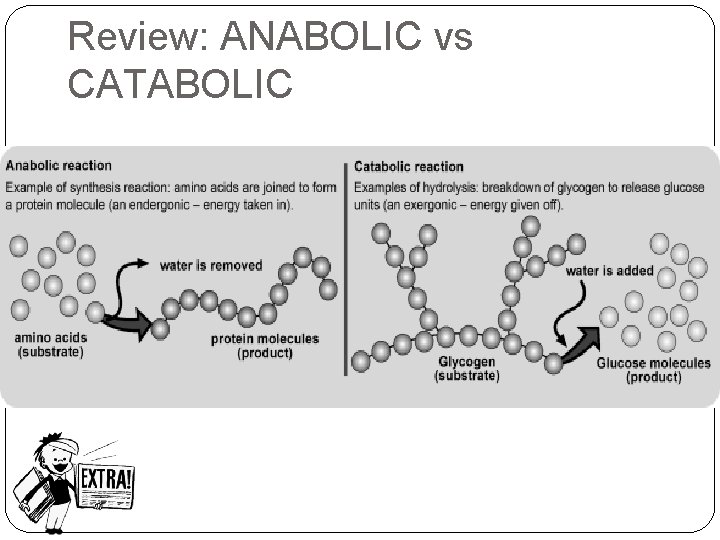
Review: ANABOLIC vs CATABOLIC
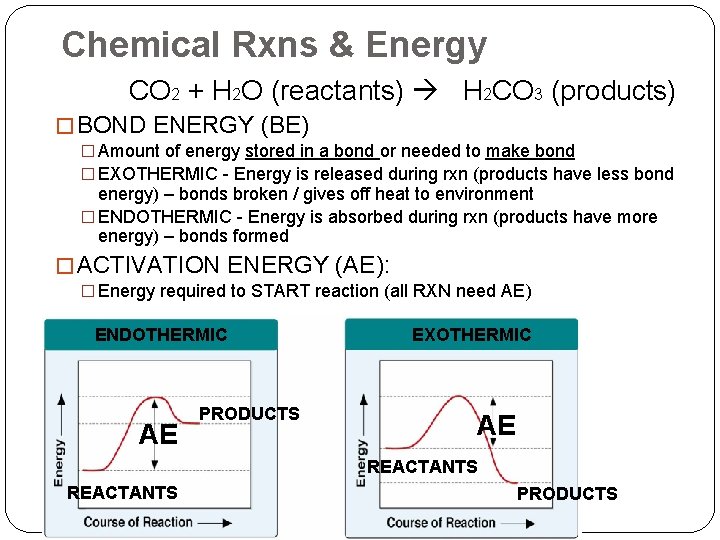
Chemical Rxns & Energy CO 2 + H 2 O (reactants) H 2 CO 3 (products) � BOND ENERGY (BE) � Amount of energy stored in a bond or needed to make bond � EXOTHERMIC - Energy is released during rxn (products have less bond energy) – bonds broken / gives off heat to environment � ENDOTHERMIC - Energy is absorbed during rxn (products have more energy) – bonds formed � ACTIVATION ENERGY (AE): � Energy required to START reaction (all RXN need AE) ENDOTHERMIC AE PRODUCTS EXOTHERMIC AE REACTANTS PRODUCTS
saltwateraquariumsupplies. org O F N AI R T X E o C / l l e fac o % 0 R 7 ATE oximately W r p p A s up lid o s s a nse ke de a s M s e � l / s e z h t e r fre Ea n e h w , d ) n n a o i p s ten le � Ex u e c c e a l f o ur s M ( r s a l d � Po n bon ge o t, r a d e y H H � ific c s) e e p s S a b h s& d �Hig i c a ( nt e v l o �S f o % 5 7 vers
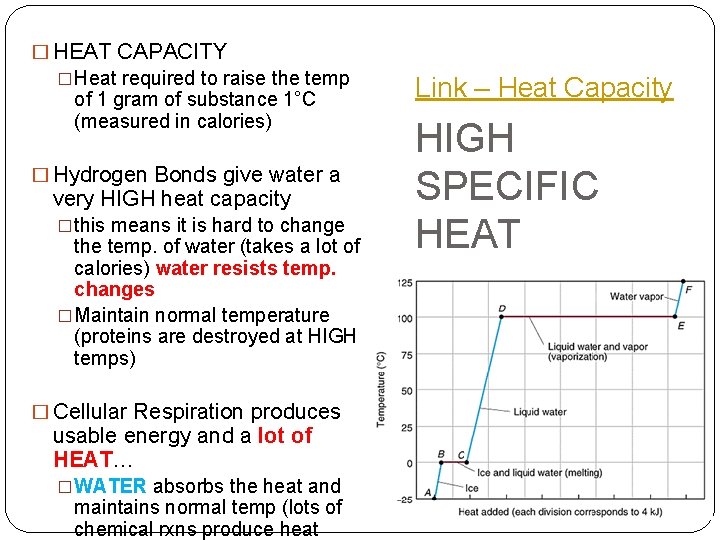
� HEAT CAPACITY �Heat required to raise the temp of 1 gram of substance 1°C (measured in calories) � Hydrogen Bonds give water a very HIGH heat capacity �this means it is hard to change the temp. of water (takes a lot of calories) water resists temp. changes �Maintain normal temperature (proteins are destroyed at HIGH temps) � Cellular Respiration produces usable energy and a lot of HEAT… �WATER absorbs the heat and maintains normal temp (lots of chemical rxns produce heat Link – Heat Capacity HIGH SPECIFIC HEAT
Acids, Bases, and p. H Water is referred to as the universal solvent, because lots of substance dissolve in water… some solutes release ions when dissolved in water. � The p. H scale (0 -14) measures the concentration of H+ ions in a solution � ACIDIC SOLUTIONS – more H+ ions than OH- (p. H below 7) � BASIC SOLUTIONS – more OH-ions than H+ (p. H above 7) � NEUTRAL SOLUTIONS – equal concentration of H+ and OH- (p. H 7) � HOMEOSTASIS – It is important for living things to maintain a p. H within normal limits because proteins are denatured by extreme changes in p. H �BUFFERS (weak acids or bases): React with STRONG acids or bases to prevent changes in p. H �EX: Blood plasma contains buffers which help maintain normal blood p. H 7. 35 -7. 45. Respiration produces CO 2 in blood can decrease p. H.
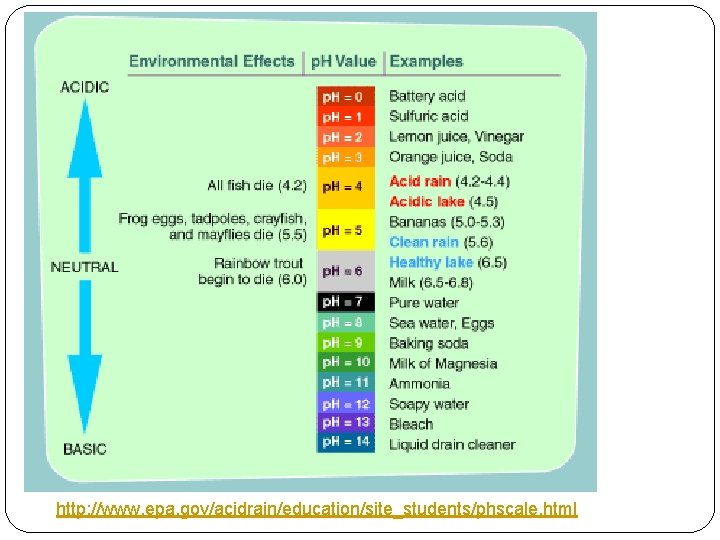
http: //www. epa. gov/acidrain/education/site_students/phscale. html
Enzymes – type of proteins �CATALYST… anything that speeds up a chemical rxn �ENZYME (organic catalyst) �(1) reduces ACTIVATION energy needed �(2) speeds up chem reaction �(3) catalyst is not change/used during rxn �PROPERTIES of enzymes: �RAPID: work quickly �REUSABLE: are not destroyed during a rxn & can be used over and over �REVERSIBLE: work in either direction (anabolic or catabolic) �SPECIFIC: only work on ONE type of rxn �“Lock & Key” (Enzyme & Substrate)
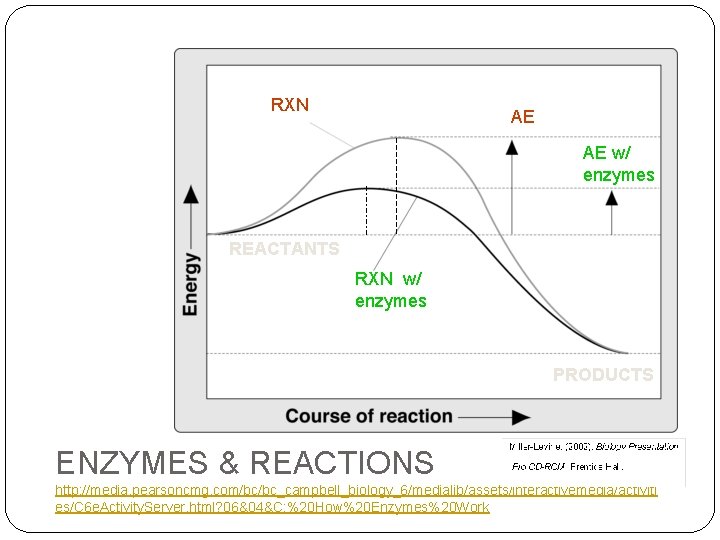
RXN AE AE w/ enzymes REACTANTS RXN w/ enzymes PRODUCTS ENZYMES & REACTIONS http: //media. pearsoncmg. com/bc/bc_campbell_biology_6/medialib/assets/interactivemedia/activiti es/C 6 e. Activity. Server. html? 06&04&C: %20 How%20 Enzymes%20 Work
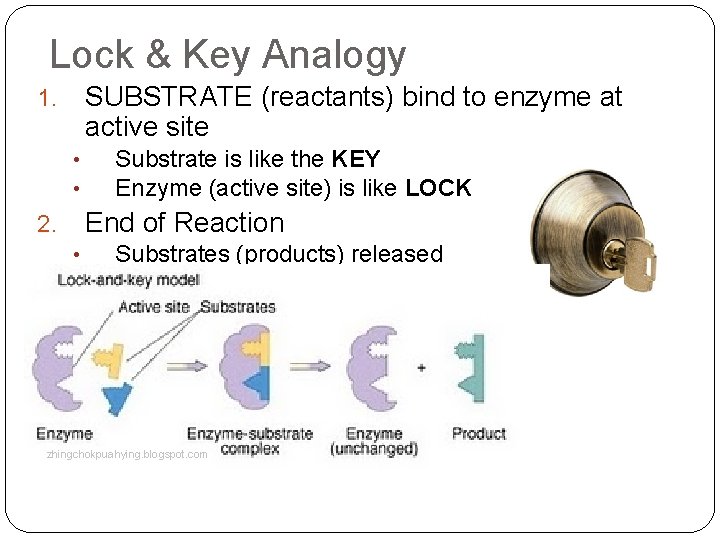
Lock & Key Analogy SUBSTRATE (reactants) bind to enzyme at active site 1. • • Substrate is like the KEY Enzyme (active site) is like LOCK End of Reaction 2. • • Substrates (products) released Enzymes reused zhingchokpuahying. blogspot. com
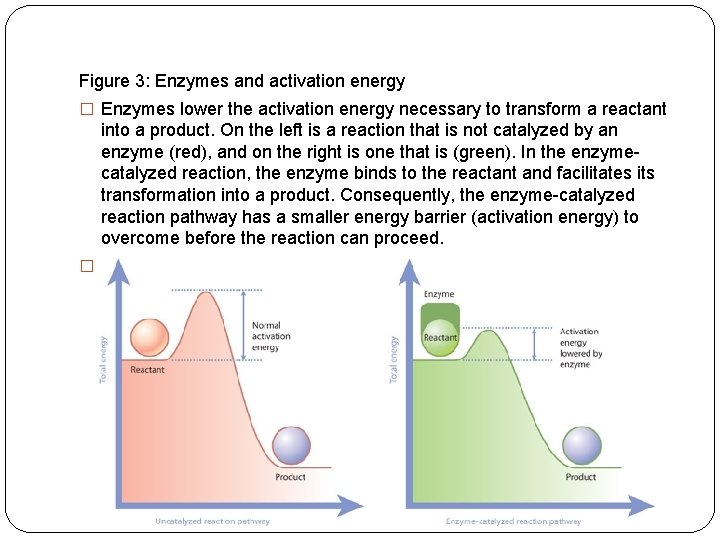
Figure 3: Enzymes and activation energy � Enzymes lower the activation energy necessary to transform a reactant into a product. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is (green). In the enzymecatalyzed reaction, the enzyme binds to the reactant and facilitates its transformation into a product. Consequently, the enzyme-catalyzed reaction pathway has a smaller energy barrier (activation energy) to overcome before the reaction can proceed. � © 2010 Nature Education All rights reserved.
- Slides: 24